Caldesmon and the regulation of cytoskeletal functions
- PMID: 19209827
- PMCID: PMC2975104
- DOI: 10.1007/978-0-387-85766-4_19
Caldesmon and the regulation of cytoskeletal functions
Abstract
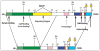
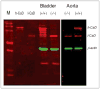
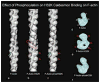
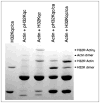
Caldesmon (CaD) is an extraordinary actin-binding protein, because in addition to actin, it also bindsmyosin, calmodulin and tropomyosin. As a component of the smoothmuscle and nonmuscle contractile apparatus CaD inhibits the actomyosin ATPase activity and its inhibitory action is modulated by both Ca2+ and phosphorylation. The multiplicity of binding partners and diverse biochemical properties suggest CaD is a potent and versatile regulatory protein both in contractility and cell motility. However, after decades ofinvestigation in numerous laboratories, hard evidence is still lacking to unequivocally identify its in vivo functions, although indirect evidence is mounting to support an important role in connection with the actin cytoskeleton. This chapter reviews the highlights of the past findings and summarizes the current views on this protein, with emphasis of its interaction with tropomyosin.
Figures


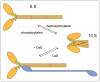
Similar articles
-
The effects of phosphorylation of smooth-muscle caldesmon.Biochem J. 1987 Jun 1;244(2):417-25. doi: 10.1042/bj2440417. Biochem J. 1987. PMID: 2822003 Free PMC article.
-
Effect of caldesmon on the position and myosin-induced movement of smooth muscle tropomyosin bound to actin.J Biol Chem. 2005 Feb 11;280(6):4135-43. doi: 10.1074/jbc.M410375200. Epub 2004 Oct 24. J Biol Chem. 2005. PMID: 15504719
-
Nonmuscle caldesmon: its distribution and involvement in various cellular processes. Review article.Protoplasma. 2004 Oct;224(1-2):1-13. doi: 10.1007/s00709-004-0057-3. Protoplasma. 2004. PMID: 15726805 Review.
-
Chapter 1: roles of caldesmon in cell motility and actin cytoskeleton remodeling.Int Rev Cell Mol Biol. 2009;274:1-68. doi: 10.1016/S1937-6448(08)02001-7. Int Rev Cell Mol Biol. 2009. PMID: 19349035 Review.
-
The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin.J Biol Chem. 1987 Jan 5;262(1):116-22. J Biol Chem. 1987. PMID: 2947901
Cited by
-
Structural studies on maturing actin filaments.Bioarchitecture. 2011 May;1(3):127-133. doi: 10.4161/bioa.1.3.16714. Bioarchitecture. 2011. PMID: 21922043 Free PMC article.
-
Identification of mitogen-activated protein kinase phosphatase-1 (MKP-1) protein partners using tandem affinity purification and mass spectrometry.Pharmacol Rep. 2023 Apr;75(2):474-481. doi: 10.1007/s43440-023-00471-7. Epub 2023 Mar 24. Pharmacol Rep. 2023. PMID: 36964420 Free PMC article.
-
Effect of l-caldesmon on osteoclastogenesis in RANKL-induced RAW264.7 cells.J Cell Physiol. 2018 Sep;233(9):6888-6901. doi: 10.1002/jcp.26452. Epub 2018 Mar 25. J Cell Physiol. 2018. PMID: 29377122 Free PMC article.
-
Desmoplastic infantile ganglioglioma with late presentation. A clinical, radiological and histopathological analysis.Neuroradiol J. 2013 Dec;26(6):649-54. doi: 10.1177/197140091302600607. Epub 2013 Dec 18. Neuroradiol J. 2013. PMID: 24355183 Free PMC article.
-
Caldesmon: Biochemical and Clinical Implications in Cancer.Front Cell Dev Biol. 2021 Feb 18;9:634759. doi: 10.3389/fcell.2021.634759. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33681215 Free PMC article. Review.
References
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
-
- Winder SJ. Structural insights into actin-binding, branching and bundling proteins. Current Opinion in Cell Biology. 2003;15:14–22. - PubMed
-
- Huber PA. Caldesmon. Int J Biochem Cell Biol. 1997;29:1047–1051. - PubMed
-
- Marston SB, Huber PAJ. Caldesmon. In: Bárány M, editor. Biochemistry of Smooth Muscle Contraction. San Diego, CA: Academic Press, Inc; 1996. pp. 77–90.
-
- Matsumura F, Yamashiro S. Caldesmon. Curr Opin Cell Biol. 1993;5:70–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

