Substrate-protein interaction in human tryptophan dioxygenase: the critical role of H76
- PMID: 19209904
- PMCID: PMC4535339
- DOI: 10.1021/ja807969a
Substrate-protein interaction in human tryptophan dioxygenase: the critical role of H76
Abstract
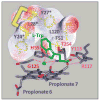
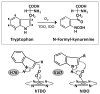
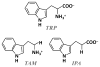
The initial and rate-limiting step of the kynurenine pathway in humans involves the oxidation of tryptophan to N-formyl kynurenine catalyzed by two hemeproteins, tryptophan 2,3-dioxygenase (hTDO) and indoleamine 2,3-dioxygenase (hIDO). In hTDO, the conserved H76 residue is believed to act as an active site base to deprotonate the indole NH group of Trp, the initial step of the Trp oxidation reaction. In hIDO, this histidine is replaced by a serine. To investigate the role of the H76, we have studied the H76S and H76A mutants of hTDO. Activity assays show that the mutations cause a decrease in k(cat) and an increase in K(M) for both mutants. The decrease in the k(cat) is accounted for by the replacement of the active site base catalyst, H76, with a weaker base, possibly a water, whereas the increase in K(M) is attributed to the loss of the specific interactions between the H76 and the substrate as well as the protein matrix. Resonance Raman studies with various Trp analogs indicate that the substrate is positioned in the active site by the ammonium, carboxylate, and indole groups, via intricate H-bonding and hydrophobic interactions. This scenario is consistent with the observation that l-Trp binding significantly perturbs the electronic properties of the O(2)-adduct of hTDO. The important structural and functional roles of H76 in hTDO is underscored by the observation that the electronic configuration of the active ternary complex, l-Trp-O(2)-hTDO, is sensitive to the H76 mutations.
Figures







References
-
- Sono M, Roach MP, Coulter ED, Dawson JH. Chem Rev. 1996;96(7):2841–2888. - PubMed
-
- Hayaishi O. J Biochem (Tokyo) 1976;79:13–21. - PubMed
-
- Feigelson O, Brady FO. In: Molecular Mechanism of Oxygen Activation. Hayaishi O, editor. Academic Press; New York: 1974. pp. 87–133.
-
- Hayaishi O, Takikawa O, Yoshida R. Prog Inorg Chem. 1990;38:75.
-
- Schutz G, Feigelson P. J Biol Chem. 1972;247:5327–5332. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

