Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2
- PMID: 19209957
- PMCID: PMC2637922
- DOI: 10.1371/journal.pbio.1000038
Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2
Abstract
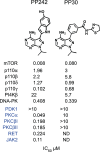
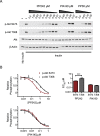
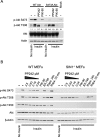
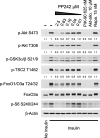
The mammalian target of rapamycin (mTOR) regulates cell growth and survival by integrating nutrient and hormonal signals. These signaling functions are distributed between at least two distinct mTOR protein complexes: mTORC1 and mTORC2. mTORC1 is sensitive to the selective inhibitor rapamycin and activated by growth factor stimulation via the canonical phosphoinositide 3-kinase (PI3K)-->Akt-->mTOR pathway. Activated mTORC1 kinase up-regulates protein synthesis by phosphorylating key regulators of mRNA translation. By contrast, mTORC2 is resistant to rapamycin. Genetic studies have suggested that mTORC2 may phosphorylate Akt at S473, one of two phosphorylation sites required for Akt activation; this has been controversial, in part because RNA interference and gene knockouts produce distinct Akt phospho-isoforms. The central role of mTOR in controlling key cellular growth and survival pathways has sparked interest in discovering mTOR inhibitors that bind to the ATP site and therefore target both mTORC2 and mTORC1. We investigated mTOR signaling in cells and animals with two novel and specific mTOR kinase domain inhibitors (TORKinibs). Unlike rapamycin, these TORKinibs (PP242 and PP30) inhibit mTORC2, and we use them to show that pharmacological inhibition of mTOR blocks the phosphorylation of Akt at S473 and prevents its full activation. Furthermore, we show that TORKinibs inhibit proliferation of primary cells more completely than rapamycin. Surprisingly, we find that mTORC2 is not the basis for this enhanced activity, and we show that the TORKinib PP242 is a more effective mTORC1 inhibitor than rapamycin. Importantly, at the molecular level, PP242 inhibits cap-dependent translation under conditions in which rapamycin has no effect. Our findings identify new functional features of mTORC1 that are resistant to rapamycin but are effectively targeted by TORKinibs. These potent new pharmacological agents complement rapamycin in the study of mTOR and its role in normal physiology and human disease.
Conflict of interest statement
Competing interests. MEF, BA, ZAK, and KMS are inventors on patent applications owned by UCSF related to PP242 and licensed to Intellikine. ZAK and KMS are consultants to Intellikine.
Figures
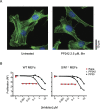
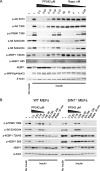
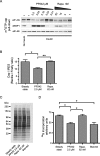

References
-
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. - PubMed
-
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. - PubMed
-
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. - PubMed
-
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. - PubMed
-
- Ruggero D, Montanaro L, Ma L, Xu W, Londei P, et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

