Neutrophils contribute to intracerebral haemorrhages after treatment with recombinant tissue plasminogen activator following cerebral ischaemia
- PMID: 19210512
- PMCID: PMC2697703
- DOI: 10.1111/j.1476-5381.2009.00068.x
Neutrophils contribute to intracerebral haemorrhages after treatment with recombinant tissue plasminogen activator following cerebral ischaemia
Abstract
Background and purpose: Polymorphonuclear neutrophils (PMNs) contribute to the vascular damage caused by transient cerebral ischaemia. Here we have evaluated the role of PMNs in intracerebral haemorrhage (ICH) induced in a model of thrombolysis with recombinant tissue plasminogen activator (t-PA) during the acute phase of cerebral ischaemia.
Experimental approach: The middle cerebral artery (MCA) of male spontaneously hypertensive rats was occluded for 1 h followed by reperfusion and, 5 h later, infusion of thrombolytic products (generated in vitro by t-PA on autologous clots). Effects of pretreatment (before the MCA occlusion) with vinblastine (4 days before; 0.5 mg.kg(-1)), monoclonal anti-neutrophil antibody (mAbRP3; 12 h, 0.3 mg.kg(-1)) or saline on ICH, neutrophil infiltration, MCA vascular reactivity and brain infarct volume were assessed, 24 h after the beginning of reperfusion.
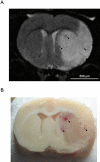
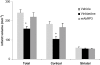
Key results: Depletion of circulating neutrophils significantly reduced t-PA-induced ICH (vinblastine, 4.6 +/- 1.0; mAbRP3, 5.2 +/- 1.0 vs. saline, 10.8 +/- 2.7 haemorrhages; P < 0.05). This depletion was associated with a decrease in cerebral infiltration by neutrophils and a decrease of endothelium-dependent, vascular dysfunction in isolated MCA, induced by the ischaemia/reperfusion and t-PA treatment. Brain infarct volume was significantly decreased after vinblastine treatment (159 +/- 13 mm(3) vs. 243 +/- 16 mm(3) with saline; P < 0.01) but not after depletion with mAbRP3 (221 +/- 22 mm(3)).
Conclusions and implications: Our results showed that pharmacological depletion of PMNs prevented t-PA-induced ICH, in parallel with a decrease in cerebral infiltration by PMNs and a decreased endothelial dysfunction in cerebral blood vessels.
Figures

References
-
- Akopov SE, Sercombe R, Seylaz J. Leukocyte-induced acute endothelial dysfunction in middle cerebral artery in rabbits. Response to aggregating platelets. Stroke. 1994;25:2246–2252. - PubMed
-
- Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:271–277. - PubMed
-
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. - PubMed
-
- Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. - PubMed
-
- Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

