EPAC proteins transduce diverse cellular actions of cAMP
- PMID: 19210747
- PMCID: PMC2795248
- DOI: 10.1111/j.1476-5381.2008.00087.x
EPAC proteins transduce diverse cellular actions of cAMP
Abstract
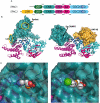
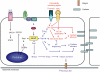
It has now been over 10 years since efforts to completely understand the signalling actions of cAMP (3'-5'-cyclic adenosine monophosphate) led to the discovery of exchange protein directly activated by cAMP (EPAC) proteins. In the current review we will highlight important advances in the understanding of EPAC structure and function and demonstrate that EPAC proteins mediate multiple actions of cAMP in cells, revealing future targets for pharmaceutical intervention. It has been known for some time that drugs that elevate intracellular cAMP levels have proven therapeutic benefit for diseases ranging from depression to inflammation. The challenge now is to determine which of these positive actions of cAMP involve activation of EPAC-regulated signal transduction pathways. EPACs are specific guanine nucleotide exchange factors for the Ras GTPase homologues, Rap1 and Rap2, which they activate independently of the classical routes for cAMP signalling, cyclic nucleotide-gated ion channels and protein kinase A. Rather, EPAC activation is triggered by internal conformational changes induced by direct interaction with cAMP. Leading from this has been the development of EPAC-specific agonists, which has helped to delineate numerous cellular actions of cAMP that rely on subsequent activation of EPAC. These include regulation of exocytosis and the control of cell adhesion, growth, division and differentiation. Recent work also implicates EPAC in the regulation of anti-inflammatory signalling in the vascular endothelium, namely negative regulation of pro-inflammatory cytokine signalling and positive support of barrier function. Further elucidation of these important signalling mechanisms will no doubt support the development of the next generation of anti-inflammatory drugs.
Figures

References
-
- Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. - PubMed
-
- Almiron I, Chemes H. Spermatogenic onset. II. FSH modulates mitotic activity of germ and Sertoli cells in immature rats. Int J Androl. 1988;11:235–246. - PubMed
-
- Anastassiou ED, Paliogianni F, Balow JP, Yamada H, Boumpas DT. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R alpha gene expression at multiple levels. J Immunol. 1992;148:2845–2852. - PubMed
-
- Arai A, Nosaka Y, Kanda E, Yamamoto K, Miyasaka N, Miura O. Rap1 is activated by erythropoietin or interleukin-3 and is involved in regulation of beta1 integrin-mediated hematopoietic cell adhesion. J Biol Chem. 2001;276:10453–10462. - PubMed
-
- Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174:595–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

