Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials
- PMID: 19211452
- PMCID: PMC5975663
- DOI: 10.1093/jnci/djn485
Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials
Abstract
Background: Multiple lines of evidence indicate that aspirin has an antineoplastic effect in the large bowel. Randomized clinical trials have been conducted to evaluate the effectiveness of aspirin for reducing the risk of colorectal adenomas. A meta-analysis of these trials will provide more precise estimates of the aspirin effect, both overall and in subgroups.
Methods: We combined data from all randomized double-blind placebo-controlled trials that evaluated aspirin for the prevention of colorectal adenomas. We used random-effects meta-analysis to estimate risk ratios and 95% confidence intervals (CIs) for the effect of aspirin on the occurrence of adenomas and of advanced lesions (ie, tubulovillous adenomas, villous adenomas, adenomas >or=1 cm in diameter, adenomas with high-grade dysplasia, or invasive cancer). All statistical tests were two-sided.
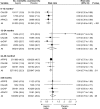
Results: We identified four clinical trials with 2967 randomly assigned participants. Each trial evaluated aspirin for the secondary prevention of colorectal adenomas. Doses of aspirin tested ranged from 81 to 325 mg/d. The average age of participants at baseline was 58 years, and 60% were male. Median follow-up was 33 months. A total of 2698 participants underwent colonoscopic follow-up and were included in the analysis of adenoma occurrence and advanced-lesion occurrence after randomization. Among these participants, adenomas were found in 424 (37%) of the 1156 participants allocated to placebo and in 507 (33%) of the 1542 participants allocated to any dose of aspirin. Advanced lesions were found in 12% of participants in the placebo group and in 9% of participants allocated to any dose of aspirin. The pooled risk ratio of any adenoma for any dose of aspirin vs placebo was 0.83 (95% CI = 0.72 to 0.96). This corresponded to an absolute risk reduction of 6.7% (95% CI = 3.2% to 10.2%). For any advanced lesion, the pooled risk ratio was 0.72 (95% CI = 0.57 to 0.90). We found no statistically significant effect modification for any of the baseline factors studied.
Conclusion: Aspirin is effective for the prevention of colorectal adenomas in individuals with a history of these lesions.
Figures




References
-
- Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. - PubMed
-
- Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–1613. - PubMed
-
- Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. New Engl J Med. 1993;328(18):1313–1316. - PubMed
-
- Labayle D, Fischer D, Vielh P, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101(3):635–639. - PubMed
-
- Rigau J, Pique JM, Rubio E, Planas R, Tarrech JM, Bordas JM. Effects of long-term sulindac therapy on colonic polyposis. Ann Internal Med. 1991;115(12):952–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

