Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5
- PMID: 19211510
- PMCID: PMC2856329
- DOI: 10.1126/scisignal.2000081
Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5
Abstract
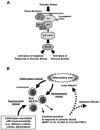
Extracellular hyperosmolarity, or osmotic stress, generally caused by differences in salt and macromolecule concentrations across the plasma membrane, occurs in lymphoid organs and at inflammatory sites. The response of immune cells to osmotic stress is regulated by nuclear factor of activated T cells 5 (NFAT5), a transcription factor that induces the expression of hyperosmolarity-responsive genes and stimulates cytokine production. We report that the guanine nucleotide exchange factor (GEF) Brx [also known as protein kinase A-anchoring protein 13 (AKAP13)] is essential for the expression of nfat5 in response to osmotic stress, thus transmitting the extracellular hyperosmolarity signal and enabling differentiation of splenic B cells and production of immunoglobulin. This process required the activity of p38 mitogen-activated protein kinase (MAPK) and NFAT5 and involved a physical interaction between Brx and c-Jun N-terminal kinase (JNK)-interacting protein 4 (JIP4), a scaffold molecule specific to activation of the p38 MAPK cascade. Our results indicate that Brx integrates the responses of immune cells to osmotic stress and inflammation by elevating intracellular osmolarity and stimulating the production of cytokines.
Figures








References
-
- Burg MB, Kwon ED, Kultz D. Regulation of gene expression by hypertonicity. Annu Rev Physiol. 1997;59:437–455. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

