Ricin A chain insertion into endoplasmic reticulum membranes is triggered by a temperature increase to 37 {degrees}C
- PMID: 19211561
- PMCID: PMC2665077
- DOI: 10.1074/jbc.M808387200
Ricin A chain insertion into endoplasmic reticulum membranes is triggered by a temperature increase to 37 {degrees}C
Abstract
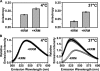
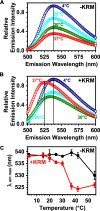
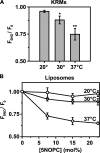
After endocytic uptake by mammalian cells, the heterodimeric plant toxin ricin is transported to the endoplasmic reticulum (ER), where the ricin A chain (RTA) must cross the ER membrane to reach its ribosomal substrates. Here, using gel filtration chromatography, sedimentation, fluorescence, fluorescence resonance energy transfer, and circular dichroism, we show that both fluorescently labeled and unlabeled RTA bind both to ER microsomal membranes and to negatively charged liposomes. The binding of RTA to the membrane at 0-30 degrees C exposes certain RTA residues to the nonpolar lipid core of the bilayer with little change in the secondary structure of the protein. However, major structural rearrangements in RTA occur when the temperature is increased. At 37 degrees C, membrane-bound toxin loses some of its helical content, and its C terminus moves closer to the membrane surface where it inserts into the bilayer. RTA is then stably bound to the membrane because it is nonextractable with carbonate. The sharp temperature dependence of the structural changes does not coincide with a lipid phase change because little change in fluorescence-detected membrane mobility occurred between 30 and 37 degrees C. Instead, the structural rearrangements may precede or initiate toxin retrotranslocation through the ER membrane to the cytosol. The sharp temperature dependence of these changes in RTA further suggests that they occur optimally in mammalian targets of the plant toxin.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

