Mechanisms underlying the control of progesterone receptor transcriptional activity by SUMOylation
- PMID: 19211567
- PMCID: PMC2666559
- DOI: 10.1074/jbc.M805226200
Mechanisms underlying the control of progesterone receptor transcriptional activity by SUMOylation
Abstract
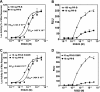
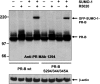
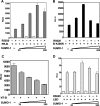
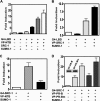
Posttranslational modification by small ubiquitin-like modifier (SUMO) is a major regulator of transcription. We previously showed that progesterone receptors (PR) have a single consensus psiKXE SUMO-conjugation motif centered at Lys-388 in the N-terminal domain of PR-B and a homologous site of PR-A. SUMOylation of the PR is hormone-dependent and has a suppressive effect on transcription of an exogenous promoter. Here we show that repression of PR activity by SUMOylation at Lys-388 is uncoupled from phosphorylation, involves synergy between tandem progesterone response elements, and is associated with lowered ligand sensitivity and slowed ligand-dependent down-regulation. However, paradoxically, cellular overexpression of SUMO-1 increases PR transcriptional activity even if Lys-388 is mutated, suggesting that the receptors are activated indirectly by other SUMOylated proteins. One of these is the coactivator SRC-1, whose binding to PR and enhancement of agonist-dependent N-/C-terminal interactions is augmented by the presence of SUMO-1. Increased transcription due to SRC-1 is independent of PR SUMOylation based on assays with the Lys-388 mutants and the pure antiprogestin ZK98299, which blocks N-/C-terminal interactions. In summary, SUMOylation tightly regulates the transcriptional activity of PR by repressing the receptors directly while activating them indirectly through augmented SRC-1 coactivation.
Figures



References
-
- Wei, L. L., Gonzalez-Aller, C., Wood, W. M., Miller, L. A., and Horwitz, K. B. (1990) Mol. Endocrinol. 4 1833–1840 - PubMed
-
- Bain, D. L., Heneghan, A. F., Connaghan-Jones, K. D., and Miura, M. T. (2007) Annu. Rev. Physiol. 69 201–220 - PubMed
-
- Sartorius, C. A., Groshong, S. D., Miller, L. A., Powell, R. L., Tung, L., Takimoto, G. S., and Horwitz, K. B. (1994) Cancer Res. 54 3868–3877 - PubMed
-
- Tung, L., Shen, T., Abel, M. G., Powell, R. L., Takimoto, G. S., Sartorius, C. A., and Horwitz, K. B. (2001) J. Biol. Chem. 276 39843–39851 - PubMed
-
- Sheridan, P. L., Francis, M. D., and Horwitz, K. B. (1989) J. Biol. Chem. 264 7054–7058 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

