Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation
- PMID: 19211680
- PMCID: PMC2727563
- DOI: 10.1242/dev.028423
Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation
Abstract
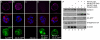
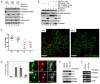
Cell-matrix adhesion is essential for the development and tissue-specific functions of epithelia. For example, in the mammary gland, beta1-integrin is necessary for the normal development of alveoli and for the activation of endocrine signalling pathways that determine cellular differentiation. However, the adhesion complex proteins linking integrins with downstream effectors of hormonal signalling pathways are not known. To understand the mechanisms involved in connecting adhesion with this aspect of cell phenotype, we examined the involvement of two proximal beta1-integrin signalling intermediates, integrin-linked kinase (ILK) and focal adhesion kinase (FAK). By employing genetic analysis using the Cre-LoxP system, we provide evidence that ILK, but not FAK, has a key role in lactogenesis in vivo and in the differentiation of cultured luminal epithelial cells. Conditional deletion of ILK both in vivo and in primary cell cultures resulted in defective differentiation, by preventing phosphorylation and nuclear translocation of STAT5, a transcription factor required for lactation. Expression of an activated RAC (RAS-related C3 botulinum substrate) in ILK-null acini restored the lactation defect, indicating that RAC1 provides a mechanistic link between the integrin/ILK adhesion complex and the differentiation pathway. Thus, we have determined that ILK is an essential downstream component of integrin signalling involved in differentiation, and have identified a high degree of specificity within the integrin-based adhesome that links cell-matrix interactions with the tissue-specific function of epithelia.
Figures



References
-
- Akhtar, N., Hudson, K. R. and Hotchin, N. A. (2000). Co-localization of Rac1 and E-cadherin in human epidermal keratinocytes. Cell Adhes. Commun. 7, 465-476. - PubMed
-
- Benard, V. and Bokoch, G. M. (2002). Assay of Cdc42, Rac, and Rho GTPase activation by affinity methods. Methods Enzymol. 345, 349-359. - PubMed
-
- Boulter, E. and Van Obberghen-Schilling, E. (2006). Integrin-linked kinase and its partners: a modular platform regulating cell-matrix adhesion dynamics and cytoskeletal organization. Eur. J. Cell Biol. 85, 255-263. - PubMed
-
- Boulter, E., Grall, D., Cagnol, S. and Van Obberghen-Schilling, E. (2006). Regulation of cell-matrix adhesion dynamics and Rac-1 by integrin linked kinase. FASEB J. 20, 1489-1491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

