Expression, localization, and function of the thioredoxin system in diabetic nephropathy
- PMID: 19211714
- PMCID: PMC2663825
- DOI: 10.1681/ASN.2008020142
Expression, localization, and function of the thioredoxin system in diabetic nephropathy
Abstract
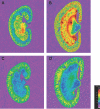
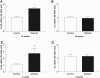
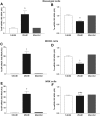
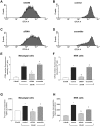
Excessive reactive oxygen species play a key role in the pathogenesis of diabetic nephropathy, but to what extent these result from increased generation, impaired antioxidant systems, or both is incompletely understood. Here, we report the expression, localization, and activity of the antioxidant thioredoxin and its endogenous inhibitor thioredoxin interacting protein (TxnIP) in vivo and in vitro. In normal human and rat kidneys, expression of TxnIP mRNA and protein was most abundant in the glomeruli and distal nephron (distal convoluted tubule and collecting ducts). In contrast, thioredoxin mRNA and protein localized to the renal cortex, particularly within the proximal tubules and to a lesser extent in the distal nephron. Induction of diabetes in rats increased expression of TxnIP but not thioredoxin mRNA. Kidneys from patients with diabetic nephropathy had significantly higher levels of TxnIP than control kidneys, but thioredoxin expression did not differ. In vitro, high glucose increased TxnIP expression in mesangial, NRK (proximal tubule), and MDCK (distal tubule/collecting duct) cells, and decreased the expression of thioredoxin in mesangial and MDCK cells. Knockdown of TxnIP with small interference RNA suggested that TxnIP mediates the glucose-induced impairment of thioredoxin activity. Knockdown of TxnIP also abrogated both glucose-induced 3H-proline incorporation (a marker of collagen production) and oxidative stress. Taken together, these findings suggest that impaired thiol reductive capacity contributes to the generation of reactive oxygen species in diabetes in a site- and cell-specific manner.
Figures





References
-
- Vaughan M: Oxidative modification of macromolecules. J Biol Chem 272: 18513, 1997
-
- Forbes JM, Coughlan MT, Cooper ME: Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 - PubMed
-
- Brownlee M: The pathobiology of diabetic complications: a unifying mechanism. Diabetes, 54: 1615–1625, 2005 - PubMed
-
- Berlett BS, Stadtman ER: Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272: 20313–20316, 1997 - PubMed
-
- Yamawaki H, Berk BC: Thioredoxin: A multifunctional antioxidant enzyme in kidney, heart and vessels. Curr Opin Nephrol Hypertens 14: 149–153, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

