Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection
- PMID: 19211743
- PMCID: PMC2668476
- DOI: 10.1128/JVI.02524-08
Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection
Abstract
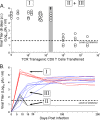
During some persistent viral infections, virus-specific T-cell responses wane due to the antigen-specific deletion or functional inactivation (i.e., exhaustion) of responding CD8 T cells. T-cell exhaustion often correlates with high viral load and is associated with the expression of the inhibitory receptor PD-1. In other infections, functional T cells are observed despite high levels of pathogen persistence. The reasons for these different T-cell fates during chronic viral infections are not clear. Here, we tracked the fate of virus-specific CD8 T cells in lymphocytic choriomeningitis virus (LCMV)-infected mice during viral clearance, the persistence of wild-type virus, or the selection and persistence of a viral variant that abrogates the presentation of a single epitope. Viral clearance results in PD-1(lo) functional virus-specific CD8 T cells, while the persistence of wild-type LCMV results in high PD-1 levels and T-cell exhaustion. However, following the emergence of a GP35V-->A variant virus that abrogates the presentation of the GP33 epitope, GP33-specific CD8 T cells remained functional, continued to show low levels of PD-1, and reexpressed CD127, a marker of memory T-cell differentiation. In the same animals and under identical environmental conditions, CD8 T cells recognizing nonmutated viral epitopes became physically deleted or were PD-1(hi) and nonfunctional. Thus, the upregulation of PD-1 and the functional inactivation of virus-specific T cells during chronic viral infection is dependent upon continued epitope recognition. These data suggest that optimal strategies for vaccination should induce high-magnitude broadly specific T-cell responses that prevent cytotoxic T-lymphocyte escape and highlight the need to evaluate the function of vaccine-induced T cells in the context of antigens presented during virus persistence.
Figures


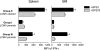

References
-
- Ahmed, R., L. A. Morrison, and D. M. Knipe. 1996. Viral persistence, p. 181-206. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, PA.
-
- Alter, G., N. Teigen, R. Ahern, H. Streeck, A. Meier, E. S. Rosenberg, and M. Altfeld. 2007. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J. Infect. Dis. 1951452-1460. - PubMed
-
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 27494-96. - PubMed
-
- Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439682-687. - PubMed
-
- Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415335-339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

