Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells
- PMID: 19211752
- PMCID: PMC2668451
- DOI: 10.1128/JVI.01910-08
Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells
Abstract
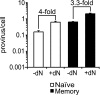
Resting CD4(+) T cells restrict human immunodeficiency virus (HIV) infection at or before reverse transcription, resulting in slower kinetics of reverse transcription. In a previous study, we showed that, despite this restriction at reverse transcription, HIV integration occurs in resting CD4(+) T cells, albeit with slower kinetics. In that study, the resting T cells were a mixture of memory and naïve cells. Here we asked whether the more quiescent naïve cell subset could be directly infected by HIV and, if so, whether the level of integration in naïve cells was comparable to that in memory cells. We found that HIV integrates in the naïve subset of resting CD4(+) T cells without prior activation of the cells. The level of integration (proviruses/cell) in naïve cells was lower than that in memory cells. This difference between naïve and memory cells was observed whether we inoculated the cells with R5 or X4 HIV and could not be explained solely by differences in coreceptor expression. The presence of endogenous dendritic cells did not change the number of proviruses/cell in memory or naïve cells, and deoxynucleoside pools were equally limiting. Our results instead indicate the existence of a novel restriction point in naïve T cells at viral fusion that results in reduced levels of fusion to naïve CD4(+) T cells. We conclude that HIV can integrate into both naïve and memory cells directly. Our data further support our hypothesis that integrated proviral infection of resting T cells can be established without T-cell activation.
Figures








References
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Ann. Rev. Immunol. 17657-700. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

