GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling
- PMID: 19211784
- PMCID: PMC2651282
- DOI: 10.1073/pnas.0900294106
GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling
Abstract
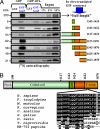
Heterotrimeric G proteins are molecular switches that control signal transduction. Ligand-occupied, G protein-coupled receptors serve as the canonical guanine nucleotide exchange factors (GEFs) that activate heterotrimeric G proteins. A few unrelated nonreceptor GEFs have also been described, but little or nothing is known about their structure, mechanism of action, or cellular functions in mammals. We have discovered that GIV/Girdin serves as a nonreceptor GEF for G alpha i through an evolutionarily conserved motif that shares sequence homology with the synthetic GEF peptide KB-752. Using the available structure of the KB-752 x G alpha i1 complex as a template, we modeled the G alpha i-GIV interface and identified the key residues that are required to form it. Mutation of these key residues disrupts the interaction and impairs Akt enhancement, actin remodeling, and cell migration in cancer cells. Mechanistically, we demonstrate that the GEF motif is capable of activating as well as sequestering the G alpha-subunit, thereby enhancing Akt signaling via the G betagamma-PI3K pathway. Recently, GIV has been implicated in cancer metastasis by virtue of its ability to enhance Akt activity and remodel the actin cytoskeleton during cancer invasion. Thus, the novel regulatory motif described here provides the structural and biochemical basis for the prometastatic features of GIV, making the functional disruption of this unique G alpha i-GIV interface a promising target for therapy against cancer metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

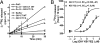

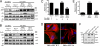
References
-
- Tall GG, Krumins AM, Gilman AG. Mammalian Ric-8A (synembryn) is a heterotrimeric Galpha protein guanine nucleotide exchange factor. J Biol Chem. 2003;278:8356–8362. - PubMed
-
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. - PubMed
-
- Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. - PubMed
-
- Cismowski MJ, et al. Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol. 1999;17:878–883. - PubMed
-
- Afshar K, et al. RIC-8 is required for GPR-1/2-dependent Galpha function during asymmetric division of C. elegans embryos. Cell. 2004;119:219–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

