TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells
- PMID: 19211797
- PMCID: PMC2651261
- DOI: 10.1073/pnas.0805323106
TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells
Abstract
Serotonin (5-hydroxytryptamine; 5-HT) is abundantly present throughout the gastrointestinal tract and stored mostly in enterochromaffin (EC) cells, which are located on the mucosal surface. 5-HT released from EC cells stimulate both intrinsic and extrinsic nerves, which results in various physiological and pathophysiological responses, such as gastrointestinal contractions. EC cells are believed to have the ability to respond to the chemical composition of the luminal contents of the gut; however, the underlying molecular and cellular mechanisms have not been identified. Here, we demonstrate that the transient receptor potential (TRP) cation channel TRPA1, which is activated by pungent compounds or cold temperature, is highly expressed in EC cells. We also found that TRPA1 agonists, including allyl isothiocyanate and cinnamaldehyde, stimulate EC cell functions, such as increasing intracellular Ca(2+) levels and 5-HT release, by using highly concentrated EC cell fractions and a model of EC cell function, the RIN14B cell line. Furthermore, we showed that allyl isothiocyanate promotes the contraction of isolated guinea pig ileum via the 5-HT(3) receptor. Taken together, our results indicate that TRPA1 acts as a sensor molecule for EC cells and may regulate gastrointestinal function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
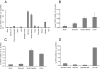
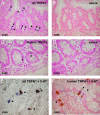
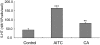
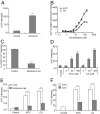


References
-
- Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54(Suppl 4):9–17. - PubMed
-
- Sundler F, Bottcher G, Ekblad E, Hakanson R. The neuroendocrine system of the gastrointestinal tract. Nord Med. 1988;103:8–11. - PubMed
-
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. - PubMed
-
- Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. - PubMed
-
- Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3:600–607. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

