In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites
- PMID: 19211879
- PMCID: PMC6666293
- DOI: 10.1523/JNEUROSCI.4249-08.2009
In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites
Abstract
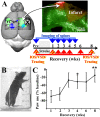
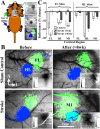
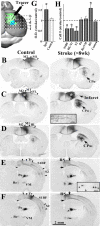
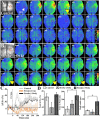
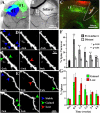
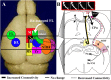
After brain damage such as stroke, topographically organized sensory and motor cortical representations remap onto adjacent surviving tissues. It is conceivable that cortical remapping is accomplished by changes in the temporal precision of sensory processing and regional connectivity in the cortex. To understand how the adult cortex remaps and processes sensory signals during stroke recovery, we performed in vivo imaging of sensory-evoked changes in membrane potential, as well as multiphoton imaging of dendrite structure and tract tracing. In control mice, forelimb stimulation evoked a brief depolarization in forelimb cortex that quickly propagated to, and dissipated within, adjacent motor/hindlimb areas (<100 ms). One week after forelimb cortex stroke, the cortex was virtually unresponsive to tactile forelimb stimulation. After 8 weeks recovery, forelimb-evoked depolarizations reemerged with a characteristic pattern in which responses began within surviving portions of forelimb cortex (<20 ms after stimulation) and then spread horizontally into neighboring peri-infarct motor/hindlimb areas in which depolarization persisted 300-400% longer than controls. These uncharacteristically prolonged responses were not limited to the remapped peri-infarct zone and included distant posteromedial retrosplenial cortex, millimeters from the stroke. Structurally, the remapped peri-infarct area selectively exhibited high levels of dendritic spine turnover, shared more connections with retrosplenial cortex and striatum, and lost inputs from lateral somatosensory cortical regions. Our findings demonstrate that sensory remapping during stroke recovery is accompanied by the development of prolonged sensory responses and new structural circuits in both the peri-infarct zone as well as more distant sites.
Figures


References
-
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol. 2006;499:33–48. - PubMed
-
- Berger T, Borgdorff A, Crochet S, Neubauer FB, Lefort S, Fauvet B, Ferezou I, Carleton A, Lüscher HR, Petersen CC. Combined voltage and calcium epifluorescence imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J Neurophysiol. 2007;97:3751–3762. - PubMed
-
- Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–999. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
