Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation
- PMID: 19211900
- PMCID: PMC6666274
- DOI: 10.1523/JNEUROSCI.5343-08.2009
Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation
Abstract
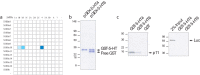
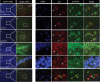
p11 (S100A10), a member of a large family of S100 proteins, interacts with serotonin receptor 1B (5-HTR1B), modulates 5-HT1B receptor signal transduction, and is required for antidepressant responses to activation of this receptor. In the current study, we investigated the specificity of the interaction between 5-HTR1B and p11 by screening brain-expressed S100 proteins against serotonin and noradrenergic receptors. The data indicate that p11 is unique among its family members for its interactions with defined serotonin receptors. We identify a novel p11-interacting receptor (5-HTR4) and characterize the interaction between p11 and 5-HTR4, demonstrating that (1) p11 and 5-HTR4 mRNA and protein are coexpressed in brain regions that are relevant for major depression, (2) p11 increases 5-HTR4 surface expression and facilitates 5-HTR4 signaling, and (3) p11 is required for the behavioral antidepressant responses to 5-HTR4 stimulation in vivo. The essential role played by p11 in modulating signaling through 5-HT4 as well as 5-HT1B receptors supports the concept that this protein may be a key determinant of vulnerability to depression.
Figures




References
-
- Attwood TK. A compendium of specific motifs for diagnosing GPCR subtypes. Trends Pharmacol Sci. 2001;22:162–165. - PubMed
-
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. - PubMed
-
- Barthet G, Framery B, Gaven F, Pellissier L, Reiter E, Claeysen S, Bockaert J, Dumuis A. 5-hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on Src activation but not on G protein or beta-arrestin signaling. Mol Biol Cell. 2007;18:1979–1991. - PMC - PubMed
-
- Bolaños-Jiménez F, Choi DS, Maroteaux L. Preferential expression of 5-HT1D over 5-HT1B receptors during early embryogenesis. Neuroreport. 1997;8:3655–3660. - PubMed
-
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
