Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells
- PMID: 19211916
- PMCID: PMC2670647
- DOI: 10.1152/ajpcell.00396.2008
Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells
Abstract
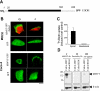
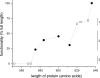
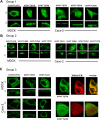
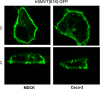
The human sodium-dependent multivitamin transporter (hSMVT) mediates sodium-dependent uptake of biotin in renal and intestinal epithelia. To date, however, there is nothing known about the structure-function relationship or targeting sequences in the hSMVT polypeptide that control its polarized expression within epithelia. Here, we focused on the role of the COOH-terminal tail of hSMVT in the targeting and functionality of this transporter. A full-length hSMVT-green fluorescent protein (GFP) fusion protein was functional and expressed at the apical membrane in renal and intestinal cell lines. Microtubule disrupting agents disrupted the mobility of trafficking vesicles and impaired cell surface delivery of hSMVT, which was also prevented in cells treated with dynamitin (p50), brefeldin, or monensin. Progressive truncation of the COOH-terminal tail impaired the functionality and targeting of the transporter. First, biotin transport decreased by approximately 20-30% on deletion of up to 15 COOH-terminal amino acids of hSMVT, a decrease mimicked solely by deletion of the terminal PDZ motif (TSL). Second, deletions into the COOH-terminal tail (between residues 584-612, containing a region of predicted high surface accessibility) resulted in a further drop in hSMVT transport (to approximately 40% of wild-type). Third, apical targeting was lost on deletion of a helical-prone region between amino acids 570-584. We conclude that the COOH tail of hSMVT contains several determinants important for polarized targeting and biotin transport.
Figures

References
-
- Anzai N, Miyazaki H, Nohiro R, Khamdang S, Chairoungdua A, Enomoto A, Hirata T, Shin HJ, Sakamoto S, Tomita K, Kanai Y, Endou H. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C-terminal. J Biol Chem 279: 45942–45950, 2004. - PubMed
-
- Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol Gastrointest Liver Physiol 285: G73–G77, 2003. - PubMed
-
- Balamurugan K, Vaziri ND, Said HM. Biotin uptake by human proximal tubular epithelial cells: cellular and molecular aspects. Am J Physiol Renal Physiol 288: F823–F831, 2005. - PubMed
-
- Banares FF, Lacruz AA, Gine JJ, Esteve M, Gassull MA. Vitamin statues in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. - PubMed
-
- Bonjour JP Vitamins and alcoholism. Int J Vitam Nutr Res 50: 321–38, 1980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

