AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells
- PMID: 19211918
- PMCID: PMC2670645
- DOI: 10.1152/ajpcell.00004.2009
AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells
Abstract
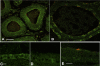
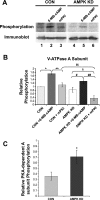
Acidic luminal pH and low [HCO(3)(-)] maintain sperm quiescent during maturation in the epididymis. The vacuolar H(+)-ATPase (V-ATPase) in clear cells is a major contributor to epididymal luminal acidification. We have shown previously that protein kinase A (PKA), acting downstream of soluble adenylyl cyclase stimulation by alkaline luminal pH or HCO(3)(-), induces V-ATPase apical membrane accumulation in clear cells. Here we examined whether the metabolic sensor AMP-activated protein kinase (AMPK) regulates this PKA-induced V-ATPase apical membrane accumulation. Immunofluorescence labeling of rat and non-human primate epididymides revealed specific AMPK expression in epithelial cells. Immunofluorescence labeling of rat epididymis showed that perfusion in vivo with the AMPK activators 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR) or A-769662 induced a redistribution of the V-ATPase into subapical vesicles, even in the presence of a luminal alkaline (pH 7.8) buffer compared with that of controls perfused without drug. Moreover, preperfusion with AICAR blocked the PKA-mediated V-ATPase translocation to clear cell apical membranes induced by N(6)-monobutyryl-cAMP (6-MB-cAMP). Purified PKA and AMPK both phosphorylated V-ATPase A subunit in vitro. In HEK-293 cells [(32)P]orthophosphate in vivo labeling of the A subunit increased following PKA stimulation and decreased following RNA interference-mediated knockdown of AMPK. Finally, the extent of PKA-dependent in vivo phosphorylation of the A subunit increased with AMPK knockdown. In summary, our findings suggest that AMPK inhibits PKA-mediated V-ATPase apical accumulation in epididymal clear cells, that both kinases directly phosphorylate the V-ATPase A subunit in vitro and in vivo, and that AMPK inhibits PKA-dependent phosphorylation of this subunit. V-ATPase activity may be coupled to the sensing of acid-base status via PKA and to metabolic status via AMPK.
Figures




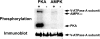

Similar articles
-
Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells.Am J Physiol Cell Physiol. 2008 Feb;294(2):C488-94. doi: 10.1152/ajpcell.00537.2007. Epub 2007 Dec 26. Am J Physiol Cell Physiol. 2008. PMID: 18160485 Free PMC article.
-
Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase.Am J Physiol Renal Physiol. 2010 May;298(5):F1162-9. doi: 10.1152/ajprenal.00645.2009. Epub 2010 Feb 10. Am J Physiol Renal Physiol. 2010. PMID: 20147366 Free PMC article.
-
Regulation of proximal tubule vacuolar H(+)-ATPase by PKA and AMP-activated protein kinase.Am J Physiol Renal Physiol. 2014 May 1;306(9):F981-95. doi: 10.1152/ajprenal.00362.2013. Epub 2014 Feb 19. Am J Physiol Renal Physiol. 2014. PMID: 24553431 Free PMC article.
-
Establishment of cell-cell cross talk in the epididymis: control of luminal acidification.J Androl. 2011 Nov-Dec;32(6):576-86. doi: 10.2164/jandrol.111.012971. Epub 2011 Mar 25. J Androl. 2011. PMID: 21441423 Free PMC article. Review.
-
H(+)V-ATPase-dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens: vesicle recycling and transcytotic pathways.J Exp Biol. 2000 Jan;203(Pt 1):137-45. doi: 10.1242/jeb.203.1.137. J Exp Biol. 2000. PMID: 10600682 Review.
Cited by
-
Role of the bicarbonate-responsive soluble adenylyl cyclase in pH sensing and metabolic regulation.Front Physiol. 2014 Feb 10;5:42. doi: 10.3389/fphys.2014.00042. eCollection 2014. Front Physiol. 2014. PMID: 24575049 Free PMC article. Review.
-
Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia.Am J Physiol Renal Physiol. 2011 Dec;301(6):F1346-57. doi: 10.1152/ajprenal.00420.2010. Epub 2011 Aug 17. Am J Physiol Renal Physiol. 2011. PMID: 21849490 Free PMC article.
-
Regulation and isoform function of the V-ATPases.Biochemistry. 2010 Jun 15;49(23):4715-23. doi: 10.1021/bi100397s. Biochemistry. 2010. PMID: 20450191 Free PMC article. Review.
-
Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action.J Clin Invest. 2015 Sep;125(9):3433-48. doi: 10.1172/JCI75245. Epub 2015 Aug 4. J Clin Invest. 2015. PMID: 26241057 Free PMC article.
-
Protein kinase A activity is necessary for fission and fusion of Golgi to endoplasmic reticulum retrograde tubules.PLoS One. 2015 Aug 10;10(8):e0135260. doi: 10.1371/journal.pone.0135260. eCollection 2015. PLoS One. 2015. PMID: 26258546 Free PMC article.
References
-
- Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem 280: 8452–8463, 2005. - PubMed
-
- Bhalla V, Daidie D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4–2 by inducing interaction with 14-3-3. Mol Endocrinol 19: 3073–3084, 2005. - PubMed
-
- Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008. - PubMed
-
- Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem 281: 26159–26169, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

