Evolution of non-coding regulatory sequences involved in the developmental process: reflection of differential employment of paralogous genes as highlighted by Sox2 and group B1 Sox genes
- PMID: 19212098
- PMCID: PMC3524295
- DOI: 10.2183/pjab.85.55
Evolution of non-coding regulatory sequences involved in the developmental process: reflection of differential employment of paralogous genes as highlighted by Sox2 and group B1 Sox genes
Abstract
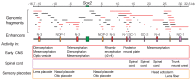
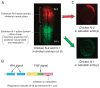
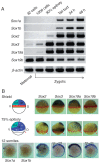
In higher vertebrates, the expression of Sox2, a group B1 Sox gene, is the hallmark of neural primordial cell state during the developmental processes from embryo to adult. Sox2 is regulated by the combined action of many enhancers with distinct spatio-temporal specificities. DNA sequences for these enhancers are conserved in a wide range of vertebrate species, corresponding to a majority of highly conserved non-coding sequences surrounding the Sox2 gene, corroborating the notion that the conservation of non-coding sequences mirrors their functional importance. Among the Sox2 enhancers, N-1 and N-2 are activated the earliest in embryogenesis and regulate Sox2 in posterior and anterior neural plates, respectively. These enhancers differ in their evolutionary history: the sequence and activity of enhancer N-2 is conserved in all vertebrate species, while enhancer N-1 is fully conserved only in amniotes. In teleost embryos, Sox19a/b play the major pan-neural role among the group B1 Sox paralogues, while strong Sox2 expression is limited to the anterior neural plate, reflecting the absence of posterior CNS-dedicated enhancers, including N-1. In Xenopus, neurally expressed SoxD is the orthologue of Sox19, but Sox3 appears to dominate other B1 paralogues. In amniotes, however, Sox19 has lost its group B1 Sox function and transforms into group G Sox15 (neofunctionalization), and Sox2 assumes the dominant position by gaining enhancer N-1 and other enhancers for posterior CNS. Thus, the gain and loss of specific enhancer elements during the evolutionary process reflects the change in functional assignment of particular paralogous genes, while overall regulatory functions attributed to the gene family are maintained.
Figures



References
-
- Hardison R. C. (2000) Conserved noncoding sequences are reliable guides to regulatory elements. Trends Genet. 16, 369–372 - PubMed
-
- Levy S., Hannenhalli S., Workman C. (2001) Enrichment of regulatory signals in conserved non-coding genomic sequence. Bioinformatics 17, 871–877 - PubMed
-
- Uchikawa M., Ishida Y., Takemoto T., Kamachi Y., Kondoh H. (2003) Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev. Cell 4, 509–519 - PubMed
-
- Uchikawa M., Takemoto T., Kamachi Y., Kondoh H. (2004) Efficient identification of regulatory sequences in the chicken genome by a powerful combination of embryo electroporation and genome comparison. Mech. Dev. 121, 1145–1158 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

