Inoculation of scrapie with the self-assembling RADA-peptide disrupts prion accumulation and extends hamster survival
- PMID: 19212437
- PMCID: PMC2636877
- DOI: 10.1371/journal.pone.0004440
Inoculation of scrapie with the self-assembling RADA-peptide disrupts prion accumulation and extends hamster survival
Abstract
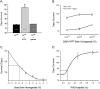
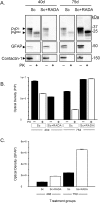
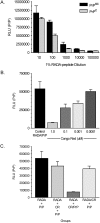
Intracerebral inoculation of 263K Scrapie brain homogenate (PrPsc) with a self-assembling RADA-peptide (RADA) significantly delayed disease onset and increased hamster survival. Time of survival was dependent on the dose of RADA and pre-incubation with PrPsc prior to inoculation. RADA treatment resulted in the absence of detectable PrPsc at 40 d followed by an increased rate of PrPsc accumulation at 75 d up to sacrifice. In all PrPsc inoculated animals, clinical symptoms were observed approximately 10 d prior to sacrifice and brains showed spongiform degeneration with Congo red positive plaques. A time-dependent increase in reactive gliosis was observed in both groups with more GFAP detected in RADA treated animals at all time points. The PrP protein showed dose-dependent binding to RADA and this binding was competitively inhibited by Congo Red. We conclude that RADA disrupts the efficacy of prion transmission by altering the rate of PrPsc accumulation. This is the first demonstration that a self-assembling biomolecular peptide can interact with PrPsc, disrupt the course of Scrapie disease process, and extend survival.
Conflict of interest statement
Figures



References
-
- Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. - PubMed
-
- DeArmond SJ, Yang SL, Cayetano-Canlas J, Groth D, Prusiner SB. The neuropathological phenotype in transgenic mice expressing different prion protein constructs. Philos Trans R Soc Lond B Biol Sci. 1994;343:415–423. - PubMed
-
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, et al. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

