Mutational analysis of the cleavage of the cancer-associated laminin receptor by stromelysin-3 reveals the contribution of flanking sequences to site recognition and cleavage efficiency
- PMID: 19212658
- PMCID: PMC2643359
- DOI: 10.3892/ijmm_00000143
Mutational analysis of the cleavage of the cancer-associated laminin receptor by stromelysin-3 reveals the contribution of flanking sequences to site recognition and cleavage efficiency
Abstract
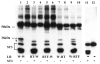
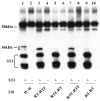
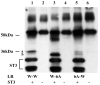
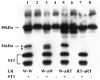
The matrix metalloproteinase stromelysin-3 (ST3) has long been implicated to play an important role in cell fate determination during normal and pathological processes. Using the thyroid hormone-dependent Xenopus laevis metamorphosis as a model, we have previously shown that ST3 is required for apoptosis during intestinal remodeling and that laminin receptor (LR) is an in vivo substrate of ST3 during this process. ST3 cleaves LR at two distinct sites that are conserved in mammalian LR. Human ST3 and LR are both associated with tumor development and cancer progression and human LR can also be cleaved by ST3, implicating a role of LR cleavage by ST3 in human cancers. Here, we carried out a series of mutational analyses on the two cleavage sites in LR. Our findings revealed that in addition to primary sequence at the cleavage site (positions P3-P3', with the cleavage occurring between P1-P1'), flanking sequences/conformation also influenced the cleavage of LR by ST3. Furthermore, alanine substitution studies led to a surprising finding that surrounding sequence and/or conformation dictated the site of cleavage in LR by ST3. These results thus have important implications in our understanding of substrate recognition and cleavage by ST3 and argue for the importance of studying ST3 cleavage in the context of full-length substrates. Furthermore, the LR cleavage mutants generated here will also be valuable tools for future studies on the role of LR cleavage by ST3 in vertebrate development and cancer progression.
Figures





Similar articles
-
Spatio-temporal regulation and cleavage by matrix metalloproteinase stromelysin-3 implicate a role for laminin receptor in intestinal remodeling during Xenopus laevis metamorphosis.Dev Dyn. 2005 Sep;234(1):190-200. doi: 10.1002/dvdy.20511. Dev Dyn. 2005. PMID: 16059908
-
The matrix metalloproteinase stromelysin-3 cleaves laminin receptor at two distinct sites between the transmembrane domain and laminin binding sequence within the extracellular domain.Cell Res. 2005 Mar;15(3):150-9. doi: 10.1038/sj.cr.7290280. Cell Res. 2005. PMID: 15780176
-
Differential regulation of cell type-specific apoptosis by stromelysin-3: a potential mechanism via the cleavage of the laminin receptor during tail resorption in Xenopus laevis.J Biol Chem. 2009 Jul 3;284(27):18545-56. doi: 10.1074/jbc.M109.017723. Epub 2009 May 8. J Biol Chem. 2009. PMID: 19429683 Free PMC article.
-
Regulation of extracellular matrix remodeling and cell fate determination by matrix metalloproteinase stromelysin-3 during thyroid hormone-dependent post-embryonic development.Pharmacol Ther. 2007 Dec;116(3):391-400. doi: 10.1016/j.pharmthera.2007.07.005. Epub 2007 Aug 31. Pharmacol Ther. 2007. PMID: 17919732 Free PMC article. Review.
-
Stromelysin-3 in the biology of the normal and neoplastic mammary gland.J Mammary Gland Biol Neoplasia. 1996 Apr;1(2):231-40. doi: 10.1007/BF02013646. J Mammary Gland Biol Neoplasia. 1996. PMID: 10887496 Review.
Cited by
-
MMP-11 promoted the oral cancer migration and Fak/Src activation.Oncotarget. 2017 May 16;8(20):32783-32793. doi: 10.18632/oncotarget.15824. Oncotarget. 2017. PMID: 28427180 Free PMC article.
-
Apoptosis in amphibian organs during metamorphosis.Apoptosis. 2010 Mar;15(3):350-64. doi: 10.1007/s10495-009-0422-y. Apoptosis. 2010. PMID: 20238476 Free PMC article. Review.
-
Impact of matrix metalloproteinase-11 gene polymorphisms upon the development and progression of hepatocellular carcinoma.Int J Med Sci. 2018 Apr 3;15(6):653-658. doi: 10.7150/ijms.23733. eCollection 2018. Int J Med Sci. 2018. PMID: 29725257 Free PMC article.
-
High level of plasma matrix metalloproteinase-11 is associated with clinicopathological characteristics in patients with oral squamous cell carcinoma.PLoS One. 2014 Nov 25;9(11):e113129. doi: 10.1371/journal.pone.0113129. eCollection 2014. PLoS One. 2014. PMID: 25423087 Free PMC article.
-
Paralogues of Mmp11 and Timp4 Interact during the Development of the Myotendinous Junction in the Zebrafish Embryo.J Dev Biol. 2019 Dec 3;7(4):22. doi: 10.3390/jdb7040022. J Dev Biol. 2019. PMID: 31816958 Free PMC article.
References
-
- Barrett JA, Rawloings ND, Woessner JF. Handbook of proteolytic enzymes. Academic Press; NY: 1998.
-
- Alexander CM, Werb Z. Extracellular matrix degradation. In: Hay ED, editor. Cell Biology of Extracellular Matrix. 2nd ed. Plenum Press; New York: 1991. pp. 255–302.
-
- Birkedal-Hansen H, Moore WGI, Bodden MK, et al. Matrix metalloproteinases: a review. Crit. Rev. in Oral Biol. and Med. 1993;4:197–250. - PubMed
-
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Current Opinion in Cell Biology. 2001;13:534–540. - PubMed
-
- Parks WC, Mecham RP. Matrix metalloproteinases. Academic Press; New York: 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

