Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata
- PMID: 19213813
- PMCID: PMC2652054
- DOI: 10.1093/jxb/ern348
Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata
Abstract
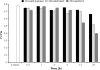
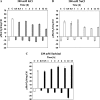
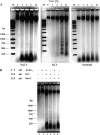
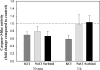
Programmed cell death (PCD) is a key element in normal plant growth and development which may also be induced by various abiotic and biotic stress factors including salt stress. In the present study, morphological, biochemical, and physiological responses of the theoretically immortal unicellular freshwater green alga Micrasterias denticulata were examined after salt (200 mM NaCl or 200 mM KCl) and osmotic stress induced by iso-osmotic sorbitol. KCl caused morphological changes such as cytoplasmic vacuolization, extreme deformation of mitochondria, and ultrastructural changes of Golgi and ER. However, prolonged salt stress (24 h) led to the degradation of organelles by autophagy, a special form of PCD, both in NaCl- and KCl-treated cells. This was indicated by the enclosure of organelles by ER-derived double membranes. DNA of NaCl- and KCl-stressed cells but not of sorbitol-treated cells showed a ladder-like pattern on agarose gel, which means that the ionic rather than the osmotic component of salt stress leads to the activation of the responsible endonuclease. DNA laddering during salt stress could be abrogated by addition of Zn(2+). Neither cytochrome c release from mitochondria nor increase in caspase-3-like activity occurred after salt stress. Reactive oxygen species could be detected within 5 min after the onset of salt and osmotic stress. Respiration, photosynthetic activity, and pigment composition indicated an active metabolism which supports programmed rather than necrotic cell death in Micrasterias after salt stress.
Figures
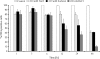

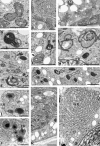
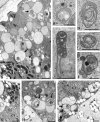
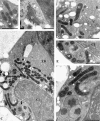
Comment in
-
Salinity and programmed cell death: unravelling mechanisms for ion specific signalling.J Exp Bot. 2009;60(3):709-12. doi: 10.1093/jxb/erp013. J Exp Bot. 2009. PMID: 19269993 No abstract available.
References
-
- Aichinger N, Lütz-Meindl U. Organelle interactions and possible degradation pathways visualized in high-pressure frozen algal cells. Journal of Microscopy. 2005;219:86–94. - PubMed
-
- Arisz SA, Valianpour F, van Gennip AH, Munnik T. Substrate preference of stress-activated phospholipase D in Chlamydomonas and its contribution to PA formation. The Plant Journal. 2003;34:595–604. - PubMed
-
- Bassham DC, Laporte M, Marty F, Moriyasu Y, Ohsumi Y, Olsen LJ, Yoshimoto K. Autophagy in development and stress responses of plants. Autophagy. 2006;2:2–11. - PubMed
-
- Bérubé KA, Dodge JD, Ford TW. Effects of chronic salt stress on the ultrastructure of Dunaliella bioculata (Chlorophyta, Volvocales): mechanisms of response and recovery. European Journal of Phycology. 1999;34:117–123.
-
- Bonneau L, Ge Y, Drury GE, Gallois P. What happened to plant caspases? Journal of Experimental Botany. 2008;59:491–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

