Imaging biological structures with fluorescence photoactivation localization microscopy
- PMID: 19214181
- PMCID: PMC2908010
- DOI: 10.1038/nprot.2008.246
Imaging biological structures with fluorescence photoactivation localization microscopy
Abstract
Fluorescence photoactivation localization microscopy (FPALM) images biological structures with subdiffraction-limited resolution. With repeated cycles of activation, readout and bleaching, large numbers of photoactivatable probes can be precisely localized to obtain a map (image) of labeled molecules with an effective resolution of tens of nanometers. FPALM has been applied to a variety of biological imaging applications, including membrane, cytoskeletal and cytosolic proteins in fixed and living cells. Molecular motions can be quantified. FPALM can also be applied to nonbiological samples, which can be labeled with photoactivatable probes. With emphasis on cellular imaging, we describe here the adaptation of a conventional widefield fluorescence microscope for FPALM and present step-by-step procedures to successfully obtain and analyze FPALM images. The fundamentals of this protocol may also be applicable to users of similar imaging techniques that apply localization of photoactivatable probes to achieve super-resolution. Once alignment of the setup has been completed, data acquisitions can be obtained in approximately 1-30 min and analyzed in approximately 0.5-4 h.
Figures

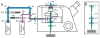




References
-
- Born M, Wolf E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light. Cambridge University Press; New York: 1997.
-
- Hell SW. Far-field optical nanoscopy. Science. 2007;316:1153–1158. - PubMed
-
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett. 1994;19:780–782. - PubMed
-
- Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

