Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females
- PMID: 19214208
- PMCID: PMC2633044
- DOI: 10.1371/journal.pgen.1000381
Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females
Erratum in
- PLoS Genet. 2009 Apr;5(4). doi: 10.1371/annotation/314060d5-06da-46e0-b9e4-57194e8ece3a doi: 10.1371/annotation/314060d5-06da-46e0-b9e4-57194e8ece3a
Abstract
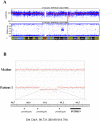
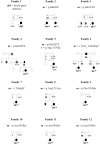
Dravet syndrome (DS) is a genetically determined epileptic encephalopathy mainly caused by de novo mutations in the SCN1A gene. Since 2003, we have performed molecular analyses in a large series of patients with DS, 27% of whom were negative for mutations or rearrangements in SCN1A. In order to identify new genes responsible for the disorder in the SCN1A-negative patients, 41 probands were screened for micro-rearrangements with Illumina high-density SNP microarrays. A hemizygous deletion on chromosome Xq22.1, encompassing the PCDH19 gene, was found in one male patient. To confirm that PCDH19 is responsible for a Dravet-like syndrome, we sequenced its coding region in 73 additional SCN1A-negative patients. Nine different point mutations (four missense and five truncating mutations) were identified in 11 unrelated female patients. In addition, we demonstrated that the fibroblasts of our male patient were mosaic for the PCDH19 deletion. Patients with PCDH19 and SCN1A mutations had very similar clinical features including the association of early febrile and afebrile seizures, seizures occurring in clusters, developmental and language delays, behavioural disturbances, and cognitive regression. There were, however, slight but constant differences in the evolution of the patients, including fewer polymorphic seizures (in particular rare myoclonic jerks and atypical absences) in those with PCDH19 mutations. These results suggest that PCDH19 plays a major role in epileptic encephalopathies, with a clinical spectrum overlapping that of DS. This disorder mainly affects females. The identification of an affected mosaic male strongly supports the hypothesis that cellular interference is the pathogenic mechanism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Stromme P, Mangelsdorf ME, Shaw MA, Lower KM, Lewis SM, et al. Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet. 2002;30:441–445. - PubMed
-
- Roll P, Rudolf G, Pereira S, Royer B, Scheffer IE, et al. SRPX2 mutations in disorders of language cortex and cognition. Hum Mol Genet. 2006;15:1195–1207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

