Recombinant bacillus Calmette-Guérin (BCG) expressing interferon-alpha 2B enhances human mononuclear cell cytotoxicity against bladder cancer cell lines in vitro
- PMID: 19214503
- PMCID: PMC11030713
- DOI: 10.1007/s00262-009-0673-z
Recombinant bacillus Calmette-Guérin (BCG) expressing interferon-alpha 2B enhances human mononuclear cell cytotoxicity against bladder cancer cell lines in vitro
Abstract
Purpose: The proper induction of cellular immunity is required for effective bacillus Calmette-Guérin (BCG) immunotherapy of bladder cancer. It has been known that BCG stimulation of human peripheral blood mononuclear cells (PBMC) leads to the generation of effector cells cytotoxic to bladder cancer cells in vitro. To improve BCG therapy, we previously developed human interferon (IFN)-alpha 2B secreting recombinant (r) BCG (rBCG-IFN-alpha). We demonstrated that rBCG-IFN-alpha augmented T helper type 1 (Th1) cytokine IFN-gamma production by PBMC. In this study, we further investigated whether rBCG-IFN-alpha could also enhance PBMC cytotoxicity toward bladder cancer cells.
Materials and methods: PBMC were prepared from healthy individuals, left alone or stimulated with rBCG-IFN-alpha or control MV261 BCG, and used as effector cells in (51)Cr-release assays. Human bladder cancer cell lines T24, J82, 5637, TCCSUP, and UMUC-3 were used as target cells. To determine the role of secreted rIFN-alpha as well as endogenously expressed IFN-gamma and IL-2 in inducing the cytotoxicity, PBMC were stimulated with rBCG-IFN-alpha in the presence of neutralizing antibodies to IFN-alpha, IFN-gamma or IL-2. To determine the role of natural killer (NK) and CD8(+) T cells in inducing the cytotoxicity, both cell types were isolated after BCG stimulation of PBMC and used as effector cells in (51)Cr-release assays.
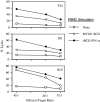
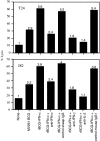
Results: Non-stimulated PBMC showed basal levels of cytotoxicity against all target cell lines tested. MV261 BCG increased the PBMC cytotoxicity by 1.8- to 4.2-fold. rBCG-IFN-alpha further increased the PBMC cytotoxicity by up to 2-fold. Elevated production of IFN-gamma and IL-2 by PBMC was observed after rBCG-IFN-alpha stimulation. Blockage of IFN-alpha, IFN-gamma or IL-2 by neutralizing antibodies during rBCG-IFN-alpha stimulation reduced or abolished the induction of PBMC cytotoxicity. Both NK and CD8(+) T cells were found to be responsible for the enhanced PBMC cytotoxicity induced by rBCG-IFN-alpha with the former cell type being more predominant.
Conclusions: rBCG-IFN-alpha is an improved BCG agent that induces enhanced PBMC cytotoxicity against bladder cancer cells in vitro. This rBCG strain may serve as an alternative to BCG for the treatment of superficial bladder cancer.
Figures





Similar articles
-
Antitumor effects of human interferon-alpha 2b secreted by recombinant bacillus Calmette-Guérin vaccine on bladder cancer cells.J Zhejiang Univ Sci B. 2012 May;13(5):335-41. doi: 10.1631/jzus.B1100366. J Zhejiang Univ Sci B. 2012. PMID: 22556170 Free PMC article.
-
Murine IL-2 secreting recombinant Bacillus Calmette-Guerin augments macrophage-mediated cytotoxicity against murine bladder cancer MBT-2.J Urol. 2000 Aug;164(2):526-31. J Urol. 2000. PMID: 10893638
-
Induction of bacillus-Calmette-Guérin-activated killer cells from human peripheral blood mononuclear cells against human bladder carcinoma cell lines in vitro.Cancer Immunol Immunother. 1993 Jul;37(2):105-11. doi: 10.1007/BF01517042. Cancer Immunol Immunother. 1993. PMID: 8319241 Free PMC article.
-
Th1 cytokine-secreting recombinant Mycobacterium bovis bacillus Calmette-Guérin and prospective use in immunotherapy of bladder cancer.Clin Dev Immunol. 2011;2011:728930. doi: 10.1155/2011/728930. Epub 2011 Sep 15. Clin Dev Immunol. 2011. PMID: 21941579 Free PMC article. Review.
-
Current state of immunotherapy for bladder cancer.Expert Rev Anticancer Ther. 2004 Dec;4(6):1037-46. doi: 10.1586/14737140.4.6.1037. Expert Rev Anticancer Ther. 2004. PMID: 15606331 Review.
Cited by
-
Future directions in bladder cancer immunotherapy: towards adaptive immunity.Immunotherapy. 2016;8(3):351-65. doi: 10.2217/imt.15.122. Epub 2016 Feb 9. Immunotherapy. 2016. PMID: 26860539 Free PMC article. Review.
-
Evolving immunotherapy strategies in urothelial cancer.Am Soc Clin Oncol Educ Book. 2015:e284-90. doi: 10.14694/EdBook_AM.2015.35.e284. Am Soc Clin Oncol Educ Book. 2015. PMID: 25993187 Free PMC article. Review.
-
Antitumor effects of human interferon-alpha 2b secreted by recombinant bacillus Calmette-Guérin vaccine on bladder cancer cells.J Zhejiang Univ Sci B. 2012 May;13(5):335-41. doi: 10.1631/jzus.B1100366. J Zhejiang Univ Sci B. 2012. PMID: 22556170 Free PMC article.
-
Intravesical therapy for urothelial carcinoma of the urinary bladder: a critical review.Int Braz J Urol. 2009 Nov-Dec;35(6):640-50; discussion 651. doi: 10.1590/s1677-55382009000600002. Int Braz J Urol. 2009. PMID: 20028569 Free PMC article. Review.
-
BCG therapy in bladder cancer and its tumor microenvironment interactions.Clin Microbiol Rev. 2025 Jun 12;38(2):e0021224. doi: 10.1128/cmr.00212-24. Epub 2025 Mar 20. Clin Microbiol Rev. 2025. PMID: 40111053 Review.
References
-
- Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette-Guérin immunotherapy of superficial bladder cancer. J Urol. 1980;124:38–40. - PubMed
-
- O’Donnell MA, DeWolf DC. Bacillus Calmette-Guérin immunotherapy for superficial bladder cancer. New prospects for an old warhorse. Surg Oncol Clin N Am. 1995;4:189–202. - PubMed
-
- Bohle A, Gerdes J, Ulmer AJ, Hofstetter AG, Flad HD. Effects of local bacillus Calmette-Guérin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J Urol. 1990;144:53–58. - PubMed
-
- Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol. 1992;147:1636–1642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

