The effect of post-irradiation tumor oxygenation status on recovery from radiation-induced damage in vivo: with reference to that in quiescent cell populations
- PMID: 19214570
- PMCID: PMC12160208
- DOI: 10.1007/s00432-009-0552-1
The effect of post-irradiation tumor oxygenation status on recovery from radiation-induced damage in vivo: with reference to that in quiescent cell populations
Abstract
Purpose: To elucidate the effect of tumor oxygenation status on recovery from damage following gamma-ray or accelerated carbon ion irradiation in vivo, including in quiescent (Q) cells.
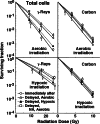
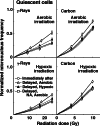
Methods: SCC VII tumor-bearing mice were continuously given 5-bromo-2'-deoxyuridine (BrdU) to label all proliferating (P) cells. They received gamma-ray or accelerated carbon ion irradiation with or without tumor clamping for inducing hypoxia. Immediately after irradiation, cells from some tumors were isolated, or acute hypoxia-releasing nicotinamide was loaded to the tumor-bearing mice. For 9 h after irradiation, some tumors were kept aerobic or hypoxic. Then isolated tumor cells were incubated with a cytokinesis blocker. The response of Q cells was assessed in terms of the micronucleus frequency using immunofluorescence staining for BrdU. That of the total (=P + Q) tumor cells was determined from BrdU non-treated tumors.
Results: Clearer recovery in Q cells than total cells and after aerobic than hypoxic gamma-ray irradiation was efficiently suppressed with carbon ion beams. Inhibition of recovery through keeping irradiated tumors hypoxic after irradiation and promotion of recovery by nicotinamide loading were observed more clearly with gamma-rays, after aerobic irradiation and in total cells than with carbon ion beams, after hypoxic irradiation and in Q cells, respectively.
Conclusions: Tumor oxygenation status following irradiation can manipulate recovery from radiation-induced damage, especially after aerobic gamma-ray irradiation in total cells. Carbon ion beams are promising because of their efficient suppression of the recovery.
Figures

References
-
- Chan N, Koritzinsky M, Zhao H, Bindra R, Glazer PM, Powell S, Belmaaza A, Wouters B, Bristow RG (2008) Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res 68:605–614. doi:10.1158/0008-5472.CAN-07-5472 - PubMed
-
- Chaplin DJ, Horsman MR, Trotter MJ (1990) Effect of nicotinamide on the microregional heterogeneity of oxygen delivery within a murine tumor. J Natl Cancer Inst 82:672–676. doi:10.1093/jnci/82.8.672 - PubMed
-
- Hada M, Georgakilas AG (2008) Formation of clustered DNA damage after high-LET irradiation: a review. J Radiat Res (Tokyo) 49:203–210. doi:10.1269/jrr.07123 - PubMed
-
- Hall EJ (2006) Repair of radiation damage and the dose-rate effect. In: Hall EJ, Giaccia AJ (eds) Radiobiology for the radiologist, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 60–84
-
- Masunaga S, Ono K (2002) Significance of the response of quiescent cell populations within solid tumors in cancer therapy. J Radiat Res (Tokyo) 43:11–25. doi:10.1269/jrr.43.11 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

