Magnetic resonance diffusion characteristics of histologically defined prostate cancer in humans
- PMID: 19215051
- PMCID: PMC3080096
- DOI: 10.1002/mrm.21896
Magnetic resonance diffusion characteristics of histologically defined prostate cancer in humans
Abstract
The contrast provided by diffusion-sensitive magnetic resonance offers the promise of improved tumor localization in organ-confined human prostate cancer (PCa). Diffusion tensor imaging (DTI) measurements of PCa were performed in vivo, in patients undergoing radical prostatectomy, and later, ex vivo, in the same patients' prostatectomy specimens. The imaging data were coregistered to histological sections of the prostatectomy specimens, thereby enabling unambiguous characterization of diffusion parameters in cancerous and benign tissues. Increased cellularity, and hence decreased luminal spaces, in peripheral zone PCa led to approximately 40% and 50% apparent diffusion policy (ADC) decrease compared with benign peripheral zone tissues in vivo and ex vivo, respectively. In contrast, no significant diffusion anisotropy differences were observed between the cancerous and noncancerous peripheral zone tissues. However, the dense fibromuscular tissues in prostate, such as stromal tissues in benign prostatic hyperplasia in central gland, exhibited high diffusion anisotropy. A tissue classification method is proposed to combine DTI and T2-weighted image contrasts that may provide improved specificity of PCa detection over T2-weighted imaging alone. PCa identified in volume rendered MR images qualitatively correlates well with histologically determined PCa foci.
Figures


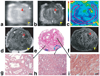
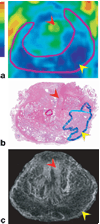
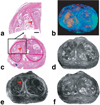
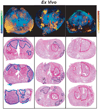
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Kantoff PW, Rarroll PR, D’Amico AV, editors. Prostate cancer: principles & practice. 1st ed. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 278–280.
-
- Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151:1571–1574. - PubMed
-
- Katz AE, Rewcastle JC. The current and potential role of cryoablation as a primary therapy for localized prostate cancer. Curr Oncol Rep. 2003;5:231–238. - PubMed
-
- Guckenberger M, Flentje M. Intensity-modulated radiotherapy (IMRT) of localized prostate cancer: a review and future perspectives. Strahlenther Onkol. 2007;183:57–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

