In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study
- PMID: 19215975
- PMCID: PMC2745073
- DOI: 10.1016/j.dental.2009.01.094
In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study
Abstract
Objective: This study explores the efficacy of an experimental orthodontic amorphous calcium phosphate (ACP) composite to remineralize in vitro subsurface enamel lesions microradiographically similar to those seen in early caries.
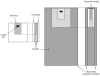
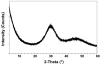
Methods: Lesions were artificially created in extracted human molars. Single tooth sections a minimum of 120microm thick were cut and individually placed in holders exposing only the carious enamel surface. The exposed surfaces were either left untreated (control) or coated with a 1mm thick layer of the experimental ACP composite (mass fraction 40% zirconia-hybridized ACP and 60% photo-activated resin), or a commercial fluoride-releasing orthodontic cement. The composite-coated sections were then photo-cured and microradiographic images were taken of all three groups of specimens before the treatment. Specimens were then cyclically immersed in demineralizing and remineralizing solutions for 1 month at 37 degrees C to simulate the pH changes occurring in the oral environment. Microradiographs of all specimens were taken before and after treatment.
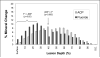
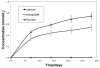
Results: Quantitative digital image analysis of matched areas from the contact microradiographs taken before and after treatment indicated higher mineral recovery with ACP composites compared to the commercial orthodontic F-releasing cement (14.4% vs. 4.3%, respectively), while the control specimens showed an average of 55.4% further demineralization.
Significance: Experimental ACP composite efficiently established mineral ion transfer throughout the body of the lesions and restored the mineral lost due to acid attack. It can be considered a useful adjuvant for the control of caries in orthodontic applications.
Figures









Similar articles
-
Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel.J Dent Res. 1996 Sep;75(9):1679-86. doi: 10.1177/00220345960750091001. J Dent Res. 1996. PMID: 8952621
-
Remineralization of demineralized enamel via calcium phosphate nanocomposite.J Dent Res. 2012 Oct;91(10):979-84. doi: 10.1177/0022034512458288. Epub 2012 Aug 29. J Dent Res. 2012. PMID: 22933607 Free PMC article.
-
Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions.J Dent Res. 1997 Sep;76(9):1587-95. doi: 10.1177/00220345970760091101. J Dent Res. 1997. PMID: 9294493
-
Remineralization and anti-demineralization effect of orthodontic adhesives on enamel surrounding orthodontic brackets: a systematic review of in vitro studies.BMC Oral Health. 2024 Nov 28;24(1):1446. doi: 10.1186/s12903-024-05237-y. BMC Oral Health. 2024. PMID: 39609782 Free PMC article.
-
Anticariogenicity of casein phosphopeptide-amorphous calcium phosphate: a review of the literature.J Contemp Dent Pract. 2009 May 1;10(3):1-9. J Contemp Dent Pract. 2009. PMID: 19430620 Review.
Cited by
-
Effect of sodium fluoride plus tricalcium phosphate with and without CO2 laser on remineralization of white spot lesions.Heliyon. 2022 Sep 30;8(10):e10752. doi: 10.1016/j.heliyon.2022.e10752. eCollection 2022 Oct. Heliyon. 2022. PMID: 36212006 Free PMC article.
-
Calcium orthophosphates (CaPO4): occurrence and properties.Prog Biomater. 2016;5:9-70. doi: 10.1007/s40204-015-0045-z. Epub 2015 Nov 19. Prog Biomater. 2016. PMID: 27471662 Free PMC article.
-
Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles.J Biomed Mater Res B Appl Biomater. 2012 Jul;100(5):1264-73. doi: 10.1002/jbm.b.32691. Epub 2012 Apr 19. J Biomed Mater Res B Appl Biomater. 2012. PMID: 22514160 Free PMC article.
-
Remineralization effectiveness of adhesive containing amorphous calcium phosphate nanoparticles on artificial initial enamel caries in a biofilm-challenged environment.Clin Oral Investig. 2021 Sep;25(9):5375-5390. doi: 10.1007/s00784-021-03846-3. Epub 2021 Apr 23. Clin Oral Investig. 2021. PMID: 33891172
-
Structural and dynamical studies of acid-mediated conversion in amorphous-calcium-phosphate based dental composites.Dent Mater. 2014 Oct;30(10):1113-25. doi: 10.1016/j.dental.2014.07.003. Epub 2014 Jul 28. Dent Mater. 2014. PMID: 25082155 Free PMC article.
References
-
- ten Cate JM, Featherstone JDB. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 1991;2(2):283–296. - PubMed
-
- ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand. 1999;57:325–329. - PubMed
-
- Kashet S. Historical review of remineralization research. J Clin Dent. 1999;10:56–64.
-
- ten Cate JM, Buijs MJ, Miller c.C., Exterkate RAM. Elevated fluoride products enhance remineralization of enamel. J Dent Res. 2008;87(10):943–947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

