Classical test theory and item response theory/Rasch model to assess differences between patient-reported fatigue using 7-day and 4-week recall periods
- PMID: 19216054
- PMCID: PMC2771583
- DOI: 10.1016/j.jclinepi.2008.10.007
Classical test theory and item response theory/Rasch model to assess differences between patient-reported fatigue using 7-day and 4-week recall periods
Abstract
Objective: This study compared self-reported fatigue between 7-day and 4-week time frames and explored factors that affect patients' responses.
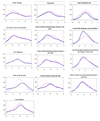
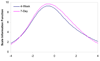
Study design and setting: Two hundred and sixteen cancer patients completed either 7-day or 4-week version of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Cochran-Mantel-Haenszel statistics and Cochran-Armitage trend tests were used to assess the association between time frame and item scores. Information function curves at both item and scale levels were depicted to evaluate the precision along the fatigue continuum. Differential item functioning (DIF) was used to examine the stability of the psychometric properties between time frames.
Results: Time frame did not influence patients' item responses. Examination of information function curves at item level did not clearly favor either time frame. At the scale level, the 7-day time frame was slightly more precise overall than the 4-week time frame. No item demonstrated DIF between time frames. Neither gender nor fatigue severity had an impact on above results.
Conclusion: This study suggests 7-day and 4-week time frame are equally appropriate in measuring fatigue, preference might be given to the more informative 7-day time frame. However, substantive considerations regarding the appropriate time frame should outweigh statistical ones.
Figures
Similar articles
-
Psychometric properties of the Chinese version of the functional assessment of chronic illness therapy-fatigue (FACIT-F) among patients with breast cancer.Health Qual Life Outcomes. 2023 Aug 15;21(1):91. doi: 10.1186/s12955-023-02164-4. Health Qual Life Outcomes. 2023. PMID: 37582752 Free PMC article.
-
Psychometric Properties of the Arabic Version of the Functional Assessment of Chronic Illnesses Therapy-Fatigue in Arabic Cancer Patients.J Pain Symptom Manage. 2020 Jan;59(1):130-138.e2. doi: 10.1016/j.jpainsymman.2019.10.008. Epub 2019 Oct 21. J Pain Symptom Manage. 2020. PMID: 31647976
-
Item banking to improve, shorten and computerize self-reported fatigue: an illustration of steps to create a core item bank from the FACIT-Fatigue Scale.Qual Life Res. 2003 Aug;12(5):485-501. doi: 10.1023/a:1025014509626. Qual Life Res. 2003. PMID: 13677494
-
Measuring fatigue in Parkinson's disease: a psychometric study of two brief generic fatigue questionnaires.J Pain Symptom Manage. 2006 Nov;32(5):420-32. doi: 10.1016/j.jpainsymman.2006.05.021. J Pain Symptom Manage. 2006. PMID: 17085268
-
Italian adaptation and psychometric validation of the Fatigue Impact Scale (FIS) and its modified versions in adults with multiple sclerosis: a Rasch analysis study.Disabil Rehabil. 2024 Nov;46(22):5366-5379. doi: 10.1080/09638288.2024.2302878. Epub 2024 Jan 18. Disabil Rehabil. 2024. PMID: 38236054
Cited by
-
GC-PROM: validation of a patient-reported outcomes measure for Chinese patients with gastric cancer.BMC Cancer. 2020 Jan 16;20(1):41. doi: 10.1186/s12885-020-6518-z. BMC Cancer. 2020. PMID: 31948422 Free PMC article.
-
Development and psychometric validation of the Pandemic-Related Traumatic Stress Scale for children and adults.Psychol Assess. 2023 Nov;35(11):1054-1067. doi: 10.1037/pas0001211. Psychol Assess. 2023. PMID: 37902671 Free PMC article.
-
Post-Intensive Care Unit Multidisciplinary Approach in Patients with Severe Bilateral SARS-CoV-2 Pneumonia.Int J Med Sci. 2023 Jan 1;20(1):1-10. doi: 10.7150/ijms.77792. eCollection 2023. Int J Med Sci. 2023. PMID: 36619225 Free PMC article.
-
Psychometric properties of the Chinese version of the functional assessment of chronic illness therapy-fatigue (FACIT-F) among patients with breast cancer.Health Qual Life Outcomes. 2023 Aug 15;21(1):91. doi: 10.1186/s12955-023-02164-4. Health Qual Life Outcomes. 2023. PMID: 37582752 Free PMC article.
-
Linking fatigue measures on a common reporting metric.J Pain Symptom Manage. 2014 Oct;48(4):639-48. doi: 10.1016/j.jpainsymman.2013.12.236. Epub 2014 Mar 31. J Pain Symptom Manage. 2014. PMID: 24698661 Free PMC article.
References
-
- North American Nursing Diagnosis Association. Nursing diagnoses: Definition and Classification, 1997–1998. Philadelphia, PA: McGraw-Hill; 1996.
-
- Cella D. Factors influencing quality of life in cancer patients: Anemia and fatigue. Semin Oncol. 1998;25(3 Suppl 7):43–46. - PubMed
-
- Cella D, Davis K, Breitbart W, Curt G. Cancer-related fatigue: Prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19(14):3385–3391. - PubMed
-
- Mooney K, Ferrell BR, Nail LM, et al. Oncology Nursing Society research priorities survey. Oncology Nursing Forum. 1991;18:1381–1388. - PubMed
-
- Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: Inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol. 2000;11(8):971–975. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical