Modulation of catalytic function by differential plasticity of the active site: case study of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase
- PMID: 19216574
- PMCID: PMC2713503
- DOI: 10.1021/bi802230y
Modulation of catalytic function by differential plasticity of the active site: case study of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase
Abstract
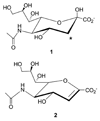
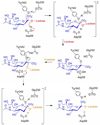
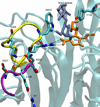
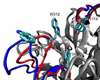
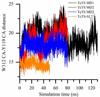
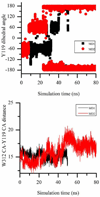
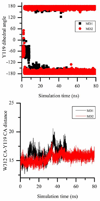
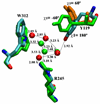
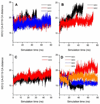
trans-Sialidase is an essential enzyme for Trypanosoma cruzi, the causative agent of Chagas' disease, to escape from the host immune system and to invade the host cells. Therefore, T. cruzi trans-sialidase (TcTS) presents a potential and appealing therapeutic target for this lethal disease. The availability of a structurally very similar enzyme with strict hydrolase activity (Trypanosoma rangeli sialidase, TrSA) provides us a unique opportunity to understand the determinants of their structure and catalytic mechanism. In this study, we compare the catalytic cleft plasticity of free (apo) and ligand-bound (holo) forms of the two enzymes using molecular dynamics simulations. We focus on the mouth of the catalytic cleft that is defined by two residues: W312 and Y119 in TcTS and W312 and S119 in TrSA. Our results indicate that TcTS has a very flexible, widely open catalytic cleft, mostly due to W312 loop motion, in apo form. However, when the catalytic cleft is occupied by a ligand, the flexibility and solvent exposure of TcTS is significantly reduced. On the other hand, TrSA maintains a more open catalytic cleft compared to its crystal structures in both apo and holo forms (and compared to TcTS in holo forms). The reduced solvent exposure of TcTS catalytic cleft might be partially or fully responsible for TcTS to be a less efficient hydrolase than TrSA.
Figures
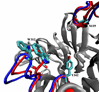

Similar articles
-
Unraveling the differences of the hydrolytic activity of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase: a quantum mechanics-molecular mechanics modeling study.J Phys Chem B. 2014 Jun 5;118(22):5807-16. doi: 10.1021/jp412294r. Epub 2014 May 21. J Phys Chem B. 2014. PMID: 24814976 Free PMC article.
-
The high resolution structures of free and inhibitor-bound Trypanosoma rangeli sialidase and its comparison with T. cruzi trans-sialidase.J Mol Biol. 2003 Jan 24;325(4):773-84. doi: 10.1016/s0022-2836(02)01306-2. J Mol Biol. 2003. PMID: 12507479
-
A sialidase mutant displaying trans-sialidase activity.J Mol Biol. 2005 Jan 28;345(4):923-34. doi: 10.1016/j.jmb.2004.09.031. J Mol Biol. 2005. PMID: 15588836
-
Trypanosoma cruzi Trans-sialidase: structural features and biological implications.Subcell Biochem. 2014;74:181-201. doi: 10.1007/978-94-007-7305-9_8. Subcell Biochem. 2014. PMID: 24264246 Review.
-
Structural and functional properties of Trypanosoma trans-sialidase.Annu Rev Microbiol. 1994;48:499-523. doi: 10.1146/annurev.mi.48.100194.002435. Annu Rev Microbiol. 1994. PMID: 7826016 Review.
Cited by
-
Carbohydrate Recognition Specificity of Trans-sialidase Lectin Domain from Trypanosoma congolense.PLoS Negl Trop Dis. 2015 Oct 16;9(10):e0004120. doi: 10.1371/journal.pntd.0004120. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26474304 Free PMC article.
-
It All Starts with a Sandwich: Identification of Sialidases with Trans-Glycosylation Activity.PLoS One. 2016 Jul 1;11(7):e0158434. doi: 10.1371/journal.pone.0158434. eCollection 2016. PLoS One. 2016. PMID: 27367145 Free PMC article.
-
Unraveling the differences of the hydrolytic activity of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase: a quantum mechanics-molecular mechanics modeling study.J Phys Chem B. 2014 Jun 5;118(22):5807-16. doi: 10.1021/jp412294r. Epub 2014 May 21. J Phys Chem B. 2014. PMID: 24814976 Free PMC article.
-
Sialic acid: a sweet swing between mammalian host and Trypanosoma cruzi.Front Immunol. 2012 Nov 29;3:356. doi: 10.3389/fimmu.2012.00356. eCollection 2012. Front Immunol. 2012. PMID: 23230438 Free PMC article.
-
Evidence of ternary complex formation in Trypanosoma cruzi trans-sialidase catalysis.J Biol Chem. 2014 Jan 3;289(1):423-36. doi: 10.1074/jbc.M112.399303. Epub 2013 Nov 5. J Biol Chem. 2014. PMID: 24194520 Free PMC article.
References
-
- Castro JA, Montalto de Mecca M, Bartel LC. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis) Hum. Exp. Toxicol. 2006;25:471–479. - PubMed
-
- Chagas C. New human trypanosomiasis. Studies about the morphology and evolutive cycle of Schizotripanum cruzi, etiological agent of a new morbid entity of man. Mem. Inst. Oswaldo Cruz. 1909;1:159–218.
-
- Kirchhoff LV. Chagas-Disease - American Trypanosomiasis. Infect. Dis. Clin. N. Am. 1993;7:487–502. - PubMed
-
- Zeledon R, Rabinovich JE. Chagas-Disease - an Ecological Appraisal with Special Emphasis on Its Insect Vectors. Annu. Rev. Entomol. 1981;26:101–133. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

