Identification of pancreatic cancer invasion-related proteins by proteomic analysis
- PMID: 19216797
- PMCID: PMC2646716
- DOI: 10.1186/1477-5956-7-3
Identification of pancreatic cancer invasion-related proteins by proteomic analysis
Abstract
Background: Markers of pancreatic cancer invasion were investigated in two clonal populations of the cell line, MiaPaCa-2, Clone #3 (high invasion) and Clone #8 (low invasion) using proteomic profiling of an in vitro model of pancreatic cancer.
Materials and methods: Using 2D-DIGE followed by MALDI-TOF MS, two clonal sub-populations of the pancreatic cancer cell line, MiaPaCa-2 with high and low invasive capacities were incubated on matrigel 24 hours prior to analysis to stimulate cell-ECM contact and mimic in vivo interaction with the basement membrane.
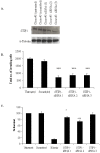
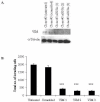
Results: Sixty proteins were identified as being differentially expressed (> 1.2 fold change and p < or = 0.05) between Clone #3 and Clone #8. Proteins found to have higher abundance levels in the highly invasive Clone #3 compared to the low invasive Clone #8 include members of the chaperone activity proteins and cytoskeleton constituents whereas metabolism-associated and catalytic proteins had lower abundance levels. Differential protein expression levels of ALDH1A1, VIM, STIP1 and KRT18 and GAPDH were confirmed by immunoblot. Using RNAi technology, STIP1 knockdown significantly reduced invasion and proliferation of the highly invasive Clone #3. Knockdown of another target, VIM by siRNA in Clone #3 cells also resulted in decreased invasion abilities of Clone #3. Elevated expression of STIP1 was observed in pancreatic tumour tissue compared to normal pancreas, whereas ALDH1A1 stained at lower levels in pancreatic tumours, as detected by immunohistochemistry.
Conclusion: Identification of targets which play a role in the highly invasive phenotype of pancreatic cancer may help to understand the biological behaviour, the rapid progression of this cancer and may be of importance in the development of new therapeutic strategies for pancreatic cancer.
Figures




Similar articles
-
Alterations in integrin expression modulates invasion of pancreatic cancer cells.J Exp Clin Cancer Res. 2009 Oct 13;28(1):140. doi: 10.1186/1756-9966-28-140. J Exp Clin Cancer Res. 2009. PMID: 19825166 Free PMC article.
-
Aldehyde dehydrogenase 1A1 and gelsolin identified as novel invasion-modulating factors in conditioned medium of pancreatic cancer cells.J Proteomics. 2008 Dec 2;71(5):561-71. doi: 10.1016/j.jprot.2008.09.002. Epub 2008 Sep 25. J Proteomics. 2008. PMID: 18848913
-
Genomic Profiling and Functional Analysis of let-7c miRNA-mRNA Interactions Identify SOX13 to Be Involved in Invasion and Progression of Pancreatic Cancer.J Oncol. 2020 Dec 24;2020:2951921. doi: 10.1155/2020/2951921. eCollection 2020. J Oncol. 2020. PMID: 33424970 Free PMC article.
-
Stress-induced phosphoprotein 1 promotes pancreatic cancer progression through activation of the FAK/AKT/MMP signaling axis.Pathol Res Pract. 2019 Nov;215(11):152564. doi: 10.1016/j.prp.2019.152564. Epub 2019 Jul 25. Pathol Res Pract. 2019. PMID: 31547977
-
KAI1 is a potential target for anti-metastasis in pancreatic cancer cells.World J Gastroenterol. 2008 Feb 21;14(7):1126-32. doi: 10.3748/wjg.14.1126. World J Gastroenterol. 2008. PMID: 18286698 Free PMC article.
Cited by
-
Phenotypic, Genomic, and Transcriptomic Heterogeneity in a Pancreatic Cancer Cell Line.Pancreas. 2024 Oct 1;53(9):e748-e759. doi: 10.1097/MPA.0000000000002371. Epub 2024 May 4. Pancreas. 2024. PMID: 38710020
-
A proteogenomic profile of early lung adenocarcinomas by protein co-expression network and genomic alteration analysis.Sci Rep. 2020 Aug 12;10(1):13604. doi: 10.1038/s41598-020-70578-x. Sci Rep. 2020. PMID: 32788598 Free PMC article.
-
Low expression of aldehyde dehydrogenase 1A1 (ALDH1A1) is a prognostic marker for poor survival in pancreatic cancer.BMC Cancer. 2011 Jun 27;11:275. doi: 10.1186/1471-2407-11-275. BMC Cancer. 2011. PMID: 21708005 Free PMC article.
-
JAK2-Mediated Phosphorylation of Stress-Induced Phosphoprotein-1 (STIP1) in Human Cells.Int J Mol Sci. 2022 Feb 22;23(5):2420. doi: 10.3390/ijms23052420. Int J Mol Sci. 2022. PMID: 35269562 Free PMC article.
-
STIP1 Regulates Proliferation and Migration of Lung Adenocarcinoma Through JAK2/STAT3 Signaling Pathway.Cancer Manag Res. 2019 Nov 29;11:10061-10072. doi: 10.2147/CMAR.S233758. eCollection 2019. Cancer Manag Res. 2019. PMID: 31819639 Free PMC article.
References
-
- Spinelli GP, Zullo A, Romiti A, Di Seri M, Tomao F, Miele E, Spalletta B, Eramo A, Hassan C, Tomao S. Long-term survival in metastatic pancreatic cancer. A case report and review of the literature. JOP. 2006;7:486–491. - PubMed
-
- Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s–5059s. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

