Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization
- PMID: 19217150
- PMCID: PMC4037868
- DOI: 10.1016/j.cell.2009.01.020
Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization
Abstract
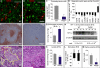
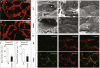
A key function of blood vessels, to supply oxygen, is impaired in tumors because of abnormalities in their endothelial lining. PHD proteins serve as oxygen sensors and may regulate oxygen delivery. We therefore studied the role of endothelial PHD2 in vessel shaping by implanting tumors in PHD2(+/-) mice. Haplodeficiency of PHD2 did not affect tumor vessel density or lumen size, but normalized the endothelial lining and vessel maturation. This resulted in improved tumor perfusion and oxygenation and inhibited tumor cell invasion, intravasation, and metastasis. Haplodeficiency of PHD2 redirected the specification of endothelial tip cells to a more quiescent cell type, lacking filopodia and arrayed in a phalanx formation. This transition relied on HIF-driven upregulation of (soluble) VEGFR-1 and VE-cadherin. Thus, decreased activity of an oxygen sensor in hypoxic conditions prompts endothelial cells to readjust their shape and phenotype to restore oxygen supply. Inhibition of PHD2 may offer alternative therapeutic opportunities for anticancer therapy.
Figures





Comment in
-
Endothelial cells form a phalanx to block tumor metastasis.Cell. 2009 Mar 6;136(5):810-2. doi: 10.1016/j.cell.2009.02.021. Cell. 2009. PMID: 19269358
-
Cancer: Suffocation of gene expression.Nature. 2016 Sep 1;537(7618):42-43. doi: 10.1038/nature19426. Epub 2016 Aug 17. Nature. 2016. PMID: 27533033 No abstract available.
References
-
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005;15:102–111. - PubMed
-
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. - PubMed
-
- Couvelard A, Deschamps L, Rebours V, Sauvanet A, Gatter K, Pezzella F, Ruszniewski P, Bedossa P. Overexpression of the oxygen sensors PHD-1, PHD-2, PHD-3, and FIH Is associated with tumor aggressiveness in pancreatic endocrine tumors. Clin. Cancer Res. 2008;14:6634–6639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

