Valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, diminishes lymphoproliferation in the Fas -deficient MRL/lpr(-/-) murine model of autoimmune lymphoproliferative syndrome (ALPS)
- PMID: 19217201
- PMCID: PMC2693256
- DOI: 10.1016/j.exphem.2008.12.002
Valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, diminishes lymphoproliferation in the Fas -deficient MRL/lpr(-/-) murine model of autoimmune lymphoproliferative syndrome (ALPS)
Abstract
Objective: Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of apoptosis, often presenting in childhood. Similarly, MRL/lpr(-/-) mice homozygous for Fas mutations develop an ALPS-like disease with autoimmunity, lymphadenopathy, splenomegaly, and expansion of double-negative T cells. Currently, there are no proven therapies with adequate safety margins for sustained abolition of the lymphoproliferation associated with ALPS. We sought to test the ability of valproic acid (VPA), a histone deacetylase inhibitor, to induce apoptosis and inhibit lymphoproliferation.
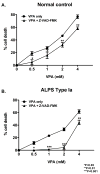
Materials and methods: Human peripheral blood mononuclear cells from patients with ALPS and normal controls were tested in vitro to determine the efficacy of VPA at inducing cell death. VPA was used in vivo to control lymphoproliferation in MRL/lpr(-/-) mice, a model for ALPS.
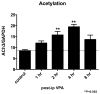
Results: VPA induced cell death in vitro, and was partially inhibited by the pan caspase inhibitor, Z-VAD-FMK. MRL/lpr(-/-) mice treated with VPA for 8 weeks showed significant reductions in spleen and lymph node weights and cellularity compared to controls. A concomitant decrease in double-negative T cells was observed in the spleen, lymph nodes, and peripheral blood. Serum levels of VPA peaked 1 hour after injection, and a 2.5-fold increase in histone acetylation was observed in the spleen at 4 hours after injection.
Conclusion: Based on our data, VPA is effective at reducing lymphoproliferation in mice, and is currently being studied in a clinical trial as a lympholytic agent in patients with ALPS.
Figures




References
-
- Lopatin U, Yao X, Williams RK, et al. Increases in circulating and lymphoid tissue interleukin-10 in autoimmune lymphoproliferative syndrome are associated with disease expression. Blood. 2001;97:3161–3170. - PubMed
-
- Sneller MC, Wang J, Dale JK, et al. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. - PubMed
-
- Rao VK. Taking ALPS down a Notch. Blood. 2008;111:477.
-
- Avila NA, Dwyer AJ, Dale JK, et al. Autoimmune lymphoproliferative syndrome: a syndrome associated with inherited genetic defects that impair lymphocytic apoptosis--CT and US features. Radiology. 1999;212:257–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

