Revisiting the modularity of modular polyketide synthases
- PMID: 19217343
- PMCID: PMC2737389
- DOI: 10.1016/j.cbpa.2008.12.018
Revisiting the modularity of modular polyketide synthases
Abstract
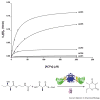
Modularity is a highly sought after feature in engineering design. A modular catalyst is a multi-component system whose parts can be predictably interchanged for functional flexibility and variety. Nearly two decades after the discovery of the first modular polyketide synthase (PKS), we critically assess PKS modularity in the face of a growing body of atomic structural and in vitro biochemical investigations. Both the architectural modularity and the functional modularity of this family of enzymatic assembly lines are reviewed, and the fundamental challenges that lie ahead for the rational exploitation of their full biosynthetic potential are discussed.
Figures



References
-
- Yoon TP, Jacobsen EN. Privileged chiral catalysts. Science. 2003;299:1691–1693. - PubMed
-
- Burk MJ. Modular Phospholane Ligands in Asymmetric Catalysis. Acc Chem Res. 2000;33:363–372. - PubMed
-
- Menzella HG, Reid R, Carney JR, Chandran SS, Reisinger SJ, Patel KG, Hopwood DA, Santi DV. Combinatorial polyketide biosynthesis by de novo design and rearrangement of modular polyketide synthase genes. Nat Biotechnol. 2005;23:1171–1176. - PubMed
-
- Kao CM, McPherson M, McDaniel R, Fu H, Cane DE, Khosla C. Alcohol stereochemistry in polyketide backbones is controlled by the beta-ketoreductase domains of modular polyketide synthases. J Am Chem Soc. 1998;120:2478–2479.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

