Labeling internalizing anti-epidermal growth factor receptor variant III monoclonal antibody with (177)Lu: in vitro comparison of acyclic and macrocyclic ligands
- PMID: 19217523
- PMCID: PMC2663407
- DOI: 10.1016/j.nucmedbio.2008.11.001
Labeling internalizing anti-epidermal growth factor receptor variant III monoclonal antibody with (177)Lu: in vitro comparison of acyclic and macrocyclic ligands
Abstract
Introduction: The monoclonal antibody (mAb) L8A4, reactive with the epidermal growth factor receptor variant III (EGFRvIII), internalizes rapidly in glioma cells after receptor binding. Combining this tumor-specific mAb with the low-energy beta-emitter (177)Lu would be an attractive approach for brain tumor radioimmunotherapy, provided that trapping of the radionuclide in tumor cells after mAb intracellular processing could be maximized.
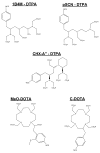
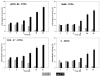
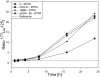
Materials and methods: L8A4 mAb was labeled with (177)Lu using the acyclic ligands [(R)-2-amino-3-(4-isothiocyanatophenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine-pentaacetic acid (CHX-A''-DTPA), 2-(4-isothiocyanatobenzyl)-diethylenetriaminepenta-acetic acid (pSCN-Bz-DTPA) and 2-(4-isothiocyanatobenzyl)-6-methyldiethylenetriaminepentaacetic acid (1B4M-DTPA), and the macrocyclic ligands S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-tetraacetic acid (C-DOTA) and alpha-(5-isothiocyanato-2-methoxyphenyl)-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (MeO-DOTA). Paired-label internalization and cellular processing assays were performed on EGFRvIII-expressing U87.DeltaEGFR glioma cells over 24 h to directly compare (177)Lu-labeled L8A4 to L8A4 labeled with (125)I using either iodogen or N-succinimidyl 4-guanidinomethyl-3-[(125)I]iodobenzoate ([(125)I]SGMIB). In order to facilitate comparison of labeling methods, the primary parameter evaluated was the ratio of (177)Lu to (125)I activity retained in U87.DeltaEGFR cells.
Results: All chelates demonstrated higher retention of internalized activity compared with mAb labeled using iodogen, with (177)Lu/(125)I ratios of >20 observed for the three DTPA chelates at 24 h. When compared to L8A4 labeled using SGMIB, except for MeO-DOTA, internalized activity for (125)I was higher than (177)Lu from 1-8 h with the opposite behavior observed thereafter. At 24 h, (177)Lu/(125)I ratios were between 1.5 and 3, with higher values observed for the three DTPA chelates.
Conclusions: The nature of the chelate used to label this internalizing mAb with (177)Lu influenced intracellular retention in vitro, although at early time points, only MeO-DOTA provided more favorable results than radioiodination of the mAb via SGMIB.
Figures



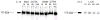
References
-
- Garcia de Palazzo IEAG, Sundareshan P, Wong AJ, Testa JR, Bigner DD, Weiner LM. Expression of Mutated Epidermal Growth Factor Receptor by Non-Small Cell Lung Carcinomas. Cancer Res. 1993;53:3217–3220. - PubMed
-
- Schwechheimer K, Huang S, Cavenee WK. EGFR gene amplification-rearrangement in human glioblastomas. Int J Cancer. 1995;62:145–148. - PubMed
-
- Antonyak MA, Moscatello DKWA. Constitutive activation of c-Jun N-terminal kinase by a mutant epidermal growth factor receptor. J Biol Chem. 1998;273:2817–2822. - PubMed
-
- Moscatello DK, Holgado-Madruga M, Emlet DR, et al. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem. 1998;273:200–206. - PubMed
-
- Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD. The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J NeuroVirol. 1998;4:148–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

