Participation of asparagine 370 and glutamine 235 in the catalysis by acid beta-glucosidase: the enzyme deficient in Gaucher disease
- PMID: 19217815
- PMCID: PMC2699545
- DOI: 10.1016/j.ymgme.2009.01.006
Participation of asparagine 370 and glutamine 235 in the catalysis by acid beta-glucosidase: the enzyme deficient in Gaucher disease
Abstract
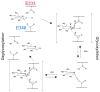
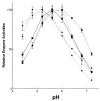
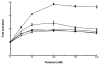
The hydrolysis of glucosylceramide by acid beta-glucosidase proceeds via a two-step, double displacement mechanism that includes cleavage of the O-beta-glucosidic bond, enzyme-glucosylation and, then, enzyme-deglucosylation. Two residues that may impact this cycle are N370 and E235. The N370S mutant enzyme is very common in Gaucher disease type 1 patients. Homology and crystal data predictions suggested that E235 is the acid/base catalyst in the hydrolytic reaction. Here, the roles of N370 and E235 in hydrolysis were explored using mutant proteins with selected amino acid substitutions. Heterologously expressed enzymes were characterized using inhibitors, activators, and alternative substrates to gain insight into the effects on the glucosylation (single turnover) and deglucosylation (transglucosylation) steps in catalysis. Specific substitutions at N370 selectively altered only the glucosylation step whereas N370S altered this and the deglucosylation steps. To provide functional data to support E235 as the acid/base catalyst, progress curves with poor substrates with more acidic leaving groups were used in the presence and absence of azide as an exogenous nucleophile. The restoration of E235G activity to nearly wild-type levels was achieved using azide with 2,4-dinitrophenyl-beta-glucoside as substrate. The loss of the acidic arm of the pH optimum activity curve of E235G provided additional functional support for E235 as the acid/base in catalysis. This study provides insight into the function of these residues in acid beta-glucosidase active site function.
Figures










Similar articles
-
Use of activators and inhibitors to define the properties of the active site of normal and Gaucher disease lysosomal beta-glucosidase.Enzyme. 1985;33(2):109-19. doi: 10.1159/000469416. Enzyme. 1985. PMID: 3924590
-
Analysis of human acid beta-glucosidase by site-directed mutagenesis and heterologous expression.J Biol Chem. 1994 Jan 21;269(3):2283-91. J Biol Chem. 1994. PMID: 8294487
-
Mechanism of the family 1 beta-glucosidase from Streptomyces sp: catalytic residues and kinetic studies.Biochemistry. 2001 May 22;40(20):5975-82. doi: 10.1021/bi002947j. Biochemistry. 2001. PMID: 11352732
-
Acid beta-glucosidase: insights from structural analysis and relevance to Gaucher disease therapy.Biol Chem. 2008 Nov;389(11):1361-9. doi: 10.1515/BC.2008.163. Biol Chem. 2008. PMID: 18783340 Review.
-
[The active site of human glucocerebrosidase: structural predictions and experimental validations].J Soc Biol. 2002;196(2):151-60. J Soc Biol. 2002. PMID: 12360744 Review. French.
Cited by
-
Biomarkers in the diagnosis of lysosomal storage disorders: proteins, lipids, and inhibodies.J Inherit Metab Dis. 2011 Jun;34(3):605-19. doi: 10.1007/s10545-011-9308-6. Epub 2011 Mar 29. J Inherit Metab Dis. 2011. PMID: 21445610 Free PMC article. Review.
-
X-ray and biochemical analysis of N370S mutant human acid β-glucosidase.J Biol Chem. 2011 Jan 7;286(1):299-308. doi: 10.1074/jbc.M110.150433. Epub 2010 Oct 27. J Biol Chem. 2011. PMID: 20980263 Free PMC article.
-
Combination of acid β-glucosidase mutation and Saposin C deficiency in mice reveals Gba1 mutation dependent and tissue-specific disease phenotype.Sci Rep. 2019 Apr 3;9(1):5571. doi: 10.1038/s41598-019-41914-7. Sci Rep. 2019. PMID: 30944381 Free PMC article.
-
Ultrasensitive in situ visualization of active glucocerebrosidase molecules.Nat Chem Biol. 2010 Dec;6(12):907-13. doi: 10.1038/nchembio.466. Epub 2010 Oct 31. Nat Chem Biol. 2010. PMID: 21079602
-
Molecular basis of reduced glucosylceramidase activity in the most common Gaucher disease mutant, N370S.J Biol Chem. 2010 Dec 31;285(53):42105-14. doi: 10.1074/jbc.M110.172098. Epub 2010 Oct 27. J Biol Chem. 2010. PMID: 20980259 Free PMC article.
References
-
- Beutler E, Grabowski GA. Gaucher disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Diseases. McGraw-Hill; New York: 2001. pp. 3635–3668.
-
- Brady RO, Kanfer JN, Shapiro D. Metabolism of glucocerebrosides. II. Evidence of an enzymatic deficiency in Gaucher’s disease. Biochem Biophys Res Commun. 1965;18:221–225. - PubMed
-
- Berg-Fussman A, Grace ME, Ioannou Y, Grabowski GA. Human β-glucosidase: N-glycosylation site occupancy and the effect of glycosylation on enzymatic activity. J Biol Chem. 1993;268:14861–14866. - PubMed
-
- Grace ME, Grabowski GA. Human acid β-glucosidase: Glycosylation is required for catalytic activity. Biochem Biophys Res Commun. 1990;168:771–777. - PubMed
-
- Erickson AH, Ginns EI, Barranger JA. Biosynthesis of the lysosomal enzyme glucocerebrosidase. J Biol Chem. 1985;260:14319–14324. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

