Transient amorphous calcium phosphate in forming enamel
- PMID: 19217943
- PMCID: PMC2731811
- DOI: 10.1016/j.jsb.2009.02.001
Transient amorphous calcium phosphate in forming enamel
Erratum in
- J Struct Biol. 2009 Jul;167(1):95
Abstract
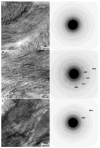
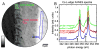
Enamel, the hardest tissue in the body, begins as a three-dimensional network of nanometer size mineral particles, suspended in a protein gel. This mineral network serves as a template for mature enamel formation. To further understand the mechanisms of enamel formation we characterized the forming enamel mineral at an early secretory stage using X-ray absorption near-edge structure (XANES) spectromicroscopy, transmission electron microscopy (TEM), FTIR microspectroscopy and polarized light microscopy. We show that the newly formed enamel mineral is amorphous calcium phosphate (ACP), which eventually transforms into apatitic crystals. Interestingly, the size, shape and spatial organization of these amorphous mineral particles and older crystals are essentially the same, indicating that the mineral morphology and organization in enamel is determined prior to its crystallization. Mineralization via transient amorphous phases has been previously reported in chiton teeth, mollusk shells, echinoderm spicules and spines, and recent reports strongly suggest the presence of transient amorphous mineral in forming vertebrate bones. The present finding of transient ACP in murine tooth enamel suggests that this strategy might be universal.
Figures


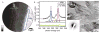


References
-
- Addadi L, Weiner S. Control and Design Principles in Biological Mineralization. Angew Chem Int Ed Engl. 1992;31:153–169.
-
- Addadi L, Beniash E, Weiner S. Assembly and Mineralization Processes in Biomineralization: Strategies for Forming Biological Composite Materials. In: Jones W, Rao CNR, editors. Supramolecular Organization and Materials Design. Cambridge University Press; Cambridge: 2002. pp. 1–34.
-
- Aizenberg J, Muller DA, Grazul JL, Hamann DR. Direct fabrication of large micropatterned single crystals. Science. 2003;299:1205–1208. - PubMed
-
- Aoba T, Moreno E. Changes in the nature and composition of enamel mineral during procine amelogenesis. Calcif Tissue Int. 1990;47:356–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

