Human cytosolic hydroxysteroid dehydrogenases of the aldo-ketoreductase superfamily catalyze reduction of conjugated steroids: implications for phase I and phase II steroid hormone metabolism
- PMID: 19218247
- PMCID: PMC2665056
- DOI: 10.1074/jbc.M809465200
Human cytosolic hydroxysteroid dehydrogenases of the aldo-ketoreductase superfamily catalyze reduction of conjugated steroids: implications for phase I and phase II steroid hormone metabolism
Abstract
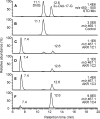
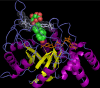
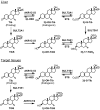
Aldo-ketoreductase 1C (AKR1C) enzymes catalyze the NADPH-dependent reduction of ketosteroids to hydroxysteroids. They are Phase I metabolizing enzymes for natural and synthetic steroid hormones. They convert 5alpha-dihydrotestosterone (Dht, potent androgen) to 3alpha/beta-androstanediols (inactive androgens) and the prodrug tibolone (Tib) to estrogenic 3alpha/beta-hydroxytibolones. Herein we demonstrate for the first time that human AKR1C enzymes (AKR1C1-4) are able to reduce conjugated steroids such as Dht-17beta-glucuronide (DhtG), Dht-17beta-sulfate (DhtS), and Tib-17beta-sulfate (TibS). Product identities were characterized by liquid chromatography-mass spectrometry, and kinetic parameters of the reactions were determined. The product profile of the reduction of each steroid conjugate by the individual AKR1C isoform was similar to that of the corresponding free steroid except for the reduction of DhtG catalyzed by AKR1C2, where a complete inversion in stereochemical preference to 3beta-reduction (with DhtG) from 3alpha-reduction (with Dht and DhtS) was observed. The catalytic efficiency of 3-keto reduction was modestly affected by the presence of a 17-sulfate group but severely impaired by the presence of a 17-glucuronide group for AKR1C1-3 isoforms. AKR1C4, however, showed superior catalytic efficiencies versus the other isoforms, and those were unaffected by steroid conjugation. Our findings provide evidence for alternative pathways of steroid metabolism where the phase I reaction (reduction) occurs after the phase II reaction (conjugation). Specifically, it is indicated that Dht is metabolized to its metabolite 3alpha-androstanediol-17-glucuronide via the previously unrecognized "conjugation pathway" involving the sequential reactions of UGT2B17 and AKR1C4 in liver but via the conventional "reduction pathway" involving the sequential reactions of AKR1C2 and UGT2B15/17 in prostate.
Figures
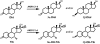



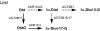

Similar articles
-
Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones.Biochem J. 2000 Oct 1;351(Pt 1):67-77. doi: 10.1042/0264-6021:3510067. Biochem J. 2000. PMID: 10998348 Free PMC article.
-
Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action.J Biol Chem. 2004 Mar 12;279(11):10784-95. doi: 10.1074/jbc.M313308200. Epub 2003 Dec 12. J Biol Chem. 2004. PMID: 14672942
-
Tibolone is metabolized by the 3alpha/3beta-hydroxysteroid dehydrogenase activities of the four human isozymes of the aldo-keto reductase 1C subfamily: inversion of stereospecificity with a delta5(10)-3-ketosteroid.Mol Pharmacol. 2004 Dec;66(6):1702-11. doi: 10.1124/mol.104.004515. Epub 2004 Sep 21. Mol Pharmacol. 2004. PMID: 15383625
-
Structure-function relationships in 3alpha-hydroxysteroid dehydrogenases: a comparison of the rat and human isoforms.J Steroid Biochem Mol Biol. 2003 Jun;85(2-5):247-55. doi: 10.1016/s0960-0760(03)00236-x. J Steroid Biochem Mol Biol. 2003. PMID: 12943710 Review.
-
Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism.Steroids. 2014 Jan;79:49-63. doi: 10.1016/j.steroids.2013.10.012. Epub 2013 Nov 1. Steroids. 2014. PMID: 24189185 Free PMC article. Review.
Cited by
-
Identification of gene co-expression clusters in liver tissues from multiple porcine populations with high and low backfat androstenone phenotype.BMC Genet. 2015 Feb 28;16:21. doi: 10.1186/s12863-014-0158-8. BMC Genet. 2015. PMID: 25884519 Free PMC article.
-
Tumor microenvironment remodeling after neoadjuvant immunotherapy in non-small cell lung cancer revealed by single-cell RNA sequencing.Genome Med. 2023 Mar 3;15(1):14. doi: 10.1186/s13073-023-01164-9. Genome Med. 2023. PMID: 36869384 Free PMC article.
-
Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review.J Steroid Biochem Mol Biol. 2019 Nov;194:105439. doi: 10.1016/j.jsbmb.2019.105439. Epub 2019 Jul 27. J Steroid Biochem Mol Biol. 2019. PMID: 31362062 Free PMC article. Review.
-
Rate of steroid double-bond reduction catalysed by the human steroid 5β-reductase (AKR1D1) is sensitive to steroid structure: implications for steroid metabolism and bile acid synthesis.Biochem J. 2014 Aug 15;462(1):163-71. doi: 10.1042/BJ20140220. Biochem J. 2014. PMID: 24894951 Free PMC article.
-
Aldo-keto reductase family 1 member C1 regulates the osteogenic differentiation of human ASCs by targeting the progesterone receptor.Stem Cell Res Ther. 2021 Jul 7;12(1):383. doi: 10.1186/s13287-021-02425-3. Stem Cell Res Ther. 2021. PMID: 34233738 Free PMC article.
References
-
- Penning, T. M. (2003) Hum. Reprod. Update 9 193-205 - PubMed
-
- Rizner, T. L., Lin, H. K., Peehl, D. M., Steckelbroeck, S., Bauman, D. R., and Penning, T. M. (2003) Endocrinology 144 2922-2932 - PubMed
-
- Steckelbroeck, S., Jin, Y., Gopishetty, S., Oyesanmi, B., and Penning, T. M. (2004) J. Biol. Chem. 279 10784-10795 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

