Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential
- PMID: 19218344
- PMCID: PMC2665769
- DOI: 10.2353/ajpath.2009.080750
Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential
Abstract
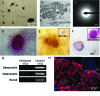
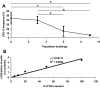
Advanced valvular lesions often contain ectopic mesenchymal tissues, which may be elaborated by an unidentified multipotent progenitor subpopulation within the valve interstitium. The identity, frequency, and differentiation potential of the putative progenitor subpopulation are unknown. The objectives of this study were to determine whether valve interstitial cells (VICs) contain a subpopulation of multipotent mesenchymal progenitor cells, to measure the frequencies of the mesenchymal progenitors and osteoprogenitors, and to characterize the osteoprogenitor subpopulation because of its potential role in calcific aortic valve disease. The multilineage potential of freshly isolated and subcultured porcine aortic VICs was tested in vitro. Progenitor frequencies and self-renewal capacity were determined by limiting dilution and colony-forming unit assays. VICs were inducible to osteogenic, adipogenic, chondrogenic, and myofibrogenic lineages. Osteogenic differentiation was also observed in situ in sclerotic porcine leaflets. Primary VICs had strikingly high frequencies of mesenchymal progenitors (48.0 +/- 5.7%) and osteoprogenitors (44.1 +/- 12.0%). High frequencies were maintained for up to six population doublings, but decreased after nine population doublings to 28.2 +/- 9.9% and 5.8 +/- 1.3%, for mesenchymal progenitors and osteoprogenitors, respectively. We further identified the putative osteoprogenitor subpopulation as morphologically distinct cells that occur at high frequency, self-renew, and elaborate bone matrix from single cells. These findings demonstrate that the aortic valve is rich in a mesenchyma l progenitor cell population that has strong potential to contribute to valve calcification.
Figures




References
-
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. - PubMed
-
- Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402 A6. - PubMed
-
- Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63–70. - PubMed
-
- Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. - PubMed
-
- Steiner I, Kasparova P, Kohout A, Dominik J. Bone formation in cardiac valves: a histopathological study of 128 cases. Virchows Arch. 2007;450:653–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

