Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes
- PMID: 19218362
- PMCID: PMC2663742
- DOI: 10.1104/pp.108.134932
Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes
Abstract
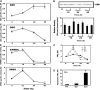
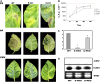
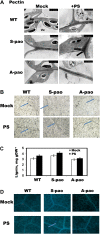
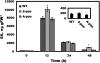
Polyamine oxidase (PAO) catalyzes the oxidative catabolism of spermidine and spermine, generating hydrogen peroxide. In wild-type tobacco (Nicotiana tabacum 'Xanthi') plants, infection by the compatible pathogen Pseudomonas syringae pv tabaci resulted in increased PAO gene and corresponding PAO enzyme activities; polyamine homeostasis was maintained by induction of the arginine decarboxylase pathway and spermine was excreted into the apoplast, where it was oxidized by the enhanced apoplastic PAO, resulting in higher hydrogen peroxide accumulation. Moreover, plants overexpressing PAO showed preinduced disease tolerance against the biotrophic bacterium P. syringae pv tabaci and the hemibiotrophic oomycete Phytophthora parasitica var nicotianae but not against the Cucumber mosaic virus. Furthermore, in transgenic PAO-overexpressing plants, systemic acquired resistance marker genes as well as a pronounced increase in the cell wall-based defense were found before inoculation. These results reveal that PAO is a nodal point in a specific apoplast-localized plant-pathogen interaction, which also signals parallel defense responses, thus preventing pathogen colonization. This strategy presents a novel approach for producing transgenic plants resistant to a broad spectrum of plant pathogens.
Figures




References
-
- Adam L, Somerville SC (1996) Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J 9 341–356 - PubMed
-
- Ahlfors R, Macioszek V, Rudd J, Broscheì M, Schlichting R, Scheel D, Kangasjarvi J (2004) Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J 40 512–522 - PubMed
-
- Alcazar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28 1867–1876 - PubMed
-
- Appiah AA, Jennings P, Turner JA (2004) Phytophthora ramorum: one pathogen and many diseases, an emerging threat to forest ecosystems and ornamental plant life. Mycologist 18 145–150
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

