CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis
- PMID: 19218364
- PMCID: PMC2689956
- DOI: 10.1104/pp.108.133272
CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis
Abstract
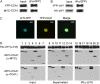
The circadian clock is an endogenous mechanism that coordinates biological processes with daily and seasonal changes in the environment. Heterodimerization of central clock components is an important way of controlling clock function in several different circadian systems. CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) are Myb-related proteins that function in or close to the central oscillator in Arabidopsis (Arabidopsis thaliana). Single mutants of cca1 and lhy have a phenotype of short-period rhythms. cca1 lhy double mutants show an even shorter period phenotype than the cca1 single mutant, suggesting that CCA1 and LHY are only partially functionally redundant. To determine whether CCA1 and LHY act in parallel or synergistically in the circadian clock, we examined their expression in both light-grown and etiolated seedlings. We have shown that LHY and CCA1 bind to the same region of the promoter of a Light-harvesting chlorophyll a/b protein (Lhcb, also known as CAB). CCA1 and LHY can form homodimers, and they also colocalize in the nucleus and heterodimerize in vitro and in vivo. In Arabidopsis, CCA1 and LHY physically interact in a manner independent of photoperiod. Moreover, results from gel filtration chromatography indicate that CCA1 and LHY are present in the same large complex in plants. Taken together, these results imply that CCA1 and LHY function synergistically in regulating circadian rhythms of Arabidopsis.
Figures





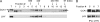
References
-
- Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Más P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293 880–883 - PubMed
-
- Alabadí D, Yanovsky MJ, Más P, Harmer SL, Kay SA (2002) Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr Biol 12 757–761 - PubMed
-
- Anderson SL, Kay SA (1997) Phototransduction and circadian clock pathways regulating gene transcription in higher plants. Adv Genet 35 1–34 - PubMed
-
- Anderson SL, Teakle GR, Martino-Catt SJ, Kay SA (1994) Circadian clock- and phytochrome-regulated transcription is conferred by a 78 bp cis-acting domain of the Arabidopsis CAB2 promoter. Plant J 6 457–470 - PubMed
-
- Carré IA, Kim JY (2002) MYB transcription factors in the Arabidopsis circadian clock. J Exp Bot 53 1551–1557 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

