SleC is essential for cortex peptidoglycan hydrolysis during germination of spores of the pathogenic bacterium Clostridium perfringens
- PMID: 19218389
- PMCID: PMC2668406
- DOI: 10.1128/JB.01832-08
SleC is essential for cortex peptidoglycan hydrolysis during germination of spores of the pathogenic bacterium Clostridium perfringens
Abstract
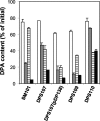
Clostridial spore germination requires degradation of the spore's peptidoglycan (PG) cortex by cortex-lytic enzymes (CLEs), and two Clostridium perfringens CLEs, SleC and SleM, degrade cortex PG in vitro. We now find that only SleC is essential for cortex hydrolysis and viability of C. perfringens spores. C. perfringens sleC spores did not germinate completely with nutrients, KCl, or a 1:1 chelate of Ca(2+) and dipicolinic acid (Ca-DPA), and the colony-forming efficiency of sleC spores was 10(3)-fold lower than that of wild-type spores. However, sleC spores incubated with various germinants released most of their DPA, although slower than wild-type or sleM spores, and DPA release from sleC sleM spores was very slow. In contrast, germination and viability of sleM spores were similar to that of wild-type spores, although sleC sleM spores had 10(5)-fold-lower viability. These results allow the following conclusions about C. perfringens spore germination: (i) SleC is essential for cortex hydrolysis; (ii) although SleM can degrade cortex PG in vitro, this enzyme is not essential; (iii) action of SleC alone or with SleM can accelerate DPA release; and (iv) Ca-DPA does not trigger spore germination by activation of CLEs.
Figures



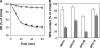
References
-
- Boland, F. M., A. Atrih, H. Chirakkal, S. J. Foster, and A. Moir. 2000. Complete spore-cortex hydrolysis during germination of Bacillus subtilis 168 requires SleB and YpeB. Microbiology 14657-64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

