p21-activated kinases Cla4 and Ste20 regulate vacuole inheritance in Saccharomyces cerevisiae
- PMID: 19218422
- PMCID: PMC2669209
- DOI: 10.1128/EC.00111-08
p21-activated kinases Cla4 and Ste20 regulate vacuole inheritance in Saccharomyces cerevisiae
Abstract
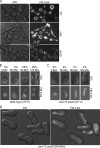
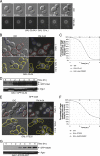
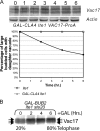
Each time Saccharomyces cerevisiae cells divide they ensure that both the mother and daughter cell inherit a vacuole by actively transporting a portion of the vacuole into the bud. As the mother cell begins budding, a tubular and vesicular segregation structure forms that is transported into the bud by the myosin V motor Myo2, which is bound to the vacuole-specific myosin receptor, Vac17 (41, 59, 70, 79). Upon arriving in the bud the segregation structure is resolved to found the daughter vacuole. The mechanism that regulates segregation structure resolution in a spatially dependent manner is unknown. In addition to resolving the segregation structure, Vac17 is degraded specifically in the bud to provide directionality to vacuole inheritance. It has been proposed that bud-specific degradation of Vac17 is promoted by proteins localized to or activated solely in the bud (77). The p21-activated kinases (PAKs) Cla4 and Ste20 are localized to and activated in the bud. Here we report that Cla4 is localized to the segregation structure just prior to segregation structure resolution, and cells lacking PAK function fail to resolve the segregation structure. Overexpression of either Cla4 or Ste20 inhibited vacuole inheritance and this inhibition was suppressed by the expression of nondegradable VAC17. Finally, PAK activity was required for Vac17 degradation in late M phase and CLA4 overexpression promoted Vac17 degradation. We propose that Cla4 and Ste20 are bud-specific proteins that play roles in both segregation structure resolution and the degradation of Vac17.
Figures




References
-
- Aitchison, J. D., M. P. Rout, M. Marelli, G. Blobel, and R. W. Wozniak. 1995. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J. Cell Biol. 1311133-1148. - PMC - PubMed
-
- Barr, F. A. 2002. Inheritance of the endoplasmic reticulum and Golgi apparatus. Curr. Opin. Cell Biol. 14496-499. - PubMed
-
- Bokoch, G. M. 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72743-781. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

