Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast
- PMID: 19218449
- PMCID: PMC2642666
- DOI: 10.1073/pnas.0813306106
Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast
Abstract
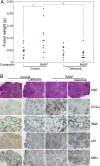
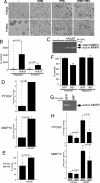
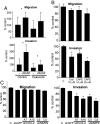
Epithelial-stromal cell interactions have an important role in breast tumor progression, but the molecular mechanisms underlying these effects are just beginning to be understood. We previously described that fibroblasts promote, whereas normal myoepithelial cells inhibit, the progression of ductal carcinoma in situ (DCIS) to invasive breast carcinomas by using a xenograft model of human DCIS. Here, we report that the tumor growth and progression-promoting effects of fibroblasts are at least in part due to increased COX-2 expression in tumor epithelial cells provoked by their interaction with fibroblasts. Up-regulation of COX-2 in DCIS xenografts resulted in increased VEGF and MMP14 expression, which may contribute to the larger weight and invasive histology of COX-2-expressing tumors. Administration of celecoxib, a selective COX-2 inhibitor, to tumor-bearing mice decreased xenograft tumor weight and inhibited progression to invasion. Coculture of fibroblasts with DCIS epithelial cells enhanced their motility and invasion, and this change was associated with increased MMP14 expression and MMP9 protease activity. We identified the NF-kappaB pathway as one of the mediators of stromal fibroblast-derived signals regulating COX-2 expression in tumor epithelial cells. Inhibition of NF-kappaB and COX-2 activity and down-regulation of MMP9 expression attenuated the invasion-promoting effects of fibroblasts. These findings support a role for COX-2 in promoting the progression of DCIS to invasive breast carcinomas, and suggest that therapeutic targeting of the NF-kappaB and prostaglandin signaling pathways might be used for the treatment and prevention of breast cancer.
Conflict of interest statement
Conflict of interest statement: K.P. receives research support from and is a consultant to Novartis Pharmaceuticals, Inc., and is also a consultant to Aveo Pharmaceuticals, Inc., and Genego Inc.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

