Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport
- PMID: 19218459
- PMCID: PMC2651306
- DOI: 10.1073/pnas.0808717106
Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport
Abstract
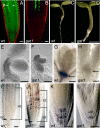
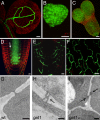
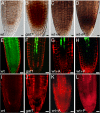
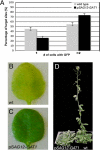
Cell-to-cell transport in plants occurs through cytoplasmic channels called "plasmodesmata" and is regulated by developmental and environmental factors. Callose deposition modulates plasmodesmal transport in vivo, but little is known about the mechanisms that regulate this process. Here we report a genetic approach to identify mutants affecting plasmodesmal transport. We isolated 5 mutants, named gfp arrested trafficking (gat), affected in GFP unloading from the phloem into the meristem. gat1 mutants were seedling lethal and carried lesions in an m-type thioredoxin that is expressed in non-green plastids of meristems and organ primordia. Callose and hydrogen peroxide accumulated in gat1 mutants, and WT plants subjected to oxidative conditions phenocopied the gat1 trafficking defects. Ectopic expression of GAT1 in mature leaves increased plasmodesmal permeability and led to a delay in senescence and flowering time. We propose a role for the GAT1 thioredoxin in the redox regulation of callose deposition and symplastic permeability that is essential for meristem maintenance in Arabidopsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Redox homeostasis regulates plasmodesmal communication in Arabidopsis meristems.Plant Signal Behav. 2009 Jul;4(7):655-9. doi: 10.1073/pnas.0808717106. Epub 2009 Jul 13. Plant Signal Behav. 2009. PMID: 19820302 Free PMC article.
References
-
- Benitez-Alfonso Y, Cantrill L, Jackson D. In: Cell-Cell Channels. Baluska F, Volkmann D, Barlow PW, editors. Austin, TX: Landes Bioscience; 2006.
-
- Lucas WJ, Lee JY. Plasmodesmata as a supracellular control network in plants. Nat Rev Mol Cell Biol. 2004;5:712–726. - PubMed
-
- Cilia M, Jackson D. Plasmodesmata form and function. Curr Opin Cell Biol. 2004;16:500–506. - PubMed
-
- Kurata T, Okada K, Wada T. Intercellular movement of transcription factors. Curr Opin Plant Biol. 2005;8:600–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

