Involution of collagen-induced arthritis with an angiogenesis inhibitor, PPI-2458
- PMID: 19218530
- PMCID: PMC2672877
- DOI: 10.1124/jpet.108.148478
Involution of collagen-induced arthritis with an angiogenesis inhibitor, PPI-2458
Abstract
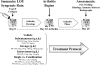
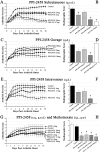
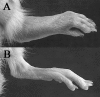
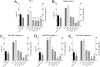
Pannus formation, in both rheumatoid arthritis (RA) and collagen-induced arthritis (CIA), is angiogenesis-dependent. PPI-2458 [(1R)-1-carbamoyl-2-methyl]-carbamic acid-(3R,3S,5S, 6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl-but-2-enyl)oxiranyl]-1-oxaspiro(2*5)oct-6-yl ester], a new fumagillin derivative known to inhibit methionine aminopeptidase 2 (MetAP-2) and endothelial proliferation at the late G(1) phase, was evaluated in CIA rats to study its potential to involute synovitis. Arthritic syngeneic LOU rats received either a vehicle control or various dosages of oral, intravenous, or subcutaneous PPI-2458. Plasma samples were analyzed to determine a pharmacokinetic profile of PPI-2458, and whole blood was evaluated by flow cytometry to assess the effect on lymphocyte subsets. At 15 mg/kg i.v., 30 mg/kg s.c., or 100 mg/kg p.o., there was a significant reduction in clinical severity scores (p < 0.001) and blinded radiographic scores (p < 0.001) compared with vehicle control groups. Structural damage was virtually eliminated with PPI-2458. Continuous inhibition of MetAP-2 was needed to maintain benefits, although pannus involution could be achieved with the inhibitor when escape flares occurred. Pharmacokinetic analysis after a single p.o. dose showed a rapid T(max) value of 15 min followed by biphasic elimination (t(1/2), approximately 20 min and t(1/2), approximately 5 h) and an estimated oral bioavailability of approximately 15%. Flow cytometry revealed a dose-dependent decrease in white blood cells and lymphocytes manifested as decreases in circulating CD3+ T cells and natural killer cells. PPI-2458, however, did not seem to be immunosuppressive, as determined by delayed-type hypersensitivity or IgG antibody assays. These studies indicate that the MetAP-2 inhibitor PPI-2458 can regress established CIA and that angiogenic mechanisms might be important targets in the treatment of other pannus-mediated diseases such as RA.
Figures



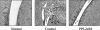

References
-
- Bendele AM, McComb J, Gould T, Frazier J, Chlipala E, Seely J, Kieft G, and Edwards CK 3rd (1999) Effects of PEGylated soluble tumor necrosis factor receptor type I (PEG sTNF-RI) alone and in combination with methotrexate in adjuvant arthritic rats. Clin Exp Rheumatol 17 553-560. - PubMed
-
- Bernier SG, Taghizadeh N, Thompson CD, Westlin WF, and Hannig G (2005) Methionine aminopeptidases type I and type II are essential to control cell proliferation. J Cell Biochem 95 1191-1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

