Interferon-gamma released by gluten-stimulated celiac disease-specific intestinal T cells enhances the transepithelial flux of gluten peptides
- PMID: 19218531
- PMCID: PMC2672868
- DOI: 10.1124/jpet.108.148007
Interferon-gamma released by gluten-stimulated celiac disease-specific intestinal T cells enhances the transepithelial flux of gluten peptides
Abstract
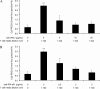
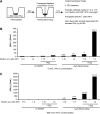
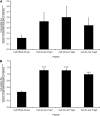
Celiac sprue is a T-cell-mediated enteropathy elicited in genetically susceptible individuals by dietary gluten proteins. To initiate and propagate inflammation, proteolytically resistant gluten peptides must be translocated across the small intestinal epithelium and presented to DQ2-restricted T cells, but the effectors enabling this translocation under normal and inflammatory conditions are not well understood. We demonstrate that a fluorescently labeled antigenic 33-mer gluten peptide is translocated intact across a T84 cultured epithelial cell monolayer and that preincubation of the monolayer with media from gluten-stimulated, celiac patient-derived intestinal T cells enhances the apical-to-basolateral flux of this peptide in a dose-dependent, saturable manner. The permeability-enhancing activity of activated T-cell media is inhibited by blocking antibodies against either interferon-gamma or its receptor and is recapitulated using recombinant interferon-gamma. At saturating levels of interferon-gamma, activated T-cell media does not further increase transepithelial peptide flux, indicating the primacy of interferon-gamma as an effector of increased epithelial permeability during inflammation. Reducing the assay temperature to 4 degrees C reverses the effect of interferon-gamma but does not reduce basal peptide flux occurring in the absence of interferon-gamma, suggesting active transcellular transport of intact peptides is increased during inflammation. A panel of disease-relevant gluten peptides exhibited an inverse correlation between size and transepithelial flux but no apparent sequence constraints. Anti-interferon-gamma therapy may mitigate the vicious cycle of gluten-induced interferon-gamma secretion and interferon-gamma-mediated enhancement of gluten peptide flux but is unlikely to prevent translocation of gluten peptides in the absence of inflammatory conditions.
Figures





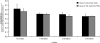

Similar articles
-
Intestinal T cell responses to gluten peptides are largely heterogeneous: implications for a peptide-based therapy in celiac disease.J Immunol. 2009 Apr 1;182(7):4158-66. doi: 10.4049/jimmunol.0803181. J Immunol. 2009. PMID: 19299713 Free PMC article.
-
Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease.Gastroenterology. 1998 Sep;115(3):551-63. doi: 10.1016/s0016-5085(98)70134-9. Gastroenterology. 1998. PMID: 9721152
-
Similar Responses of Intestinal T Cells From Untreated Children and Adults With Celiac Disease to Deamidated Gluten Epitopes.Gastroenterology. 2017 Sep;153(3):787-798.e4. doi: 10.1053/j.gastro.2017.05.016. Epub 2017 May 20. Gastroenterology. 2017. PMID: 28535873
-
Celiac disease: risk assessment, diagnosis, and monitoring.Mol Diagn Ther. 2008;12(5):289-98. doi: 10.1007/BF03256294. Mol Diagn Ther. 2008. PMID: 18803427 Review.
-
Parallels between pathogens and gluten peptides in celiac sprue.PLoS Pathog. 2008 Feb;4(2):e34. doi: 10.1371/journal.ppat.0040034. PLoS Pathog. 2008. PMID: 18425213 Free PMC article. Review.
Cited by
-
Lipid profile, atherogenic indices, and their relationship with epicardial fat thickness and carotid intima-media thickness in celiac disease.North Clin Istanb. 2019 Sep 2;6(3):242-247. doi: 10.14744/nci.2019.54936. eCollection 2019. North Clin Istanb. 2019. PMID: 31650110 Free PMC article.
-
Epithelial transport and deamidation of gliadin peptides: a role for coeliac disease patient immunoglobulin A.Clin Exp Immunol. 2011 Apr;164(1):127-36. doi: 10.1111/j.1365-2249.2010.04317.x. Epub 2011 Jan 14. Clin Exp Immunol. 2011. PMID: 21235541 Free PMC article.
-
Translational Chemistry Meets Gluten-Related Disorders.ChemistryOpen. 2018 Feb 27;7(3):217-232. doi: 10.1002/open.201700197. eCollection 2018 Mar. ChemistryOpen. 2018. PMID: 29531885 Free PMC article. Review.
-
Role of transglutaminase 2 in celiac disease pathogenesis.Semin Immunopathol. 2012 Jul;34(4):513-22. doi: 10.1007/s00281-012-0305-0. Epub 2012 Mar 22. Semin Immunopathol. 2012. PMID: 22437759 Free PMC article. Review.
-
Selected Probiotic Lactobacilli Have the Capacity To Hydrolyze Gluten Peptides during Simulated Gastrointestinal Digestion.Appl Environ Microbiol. 2017 Jun 30;83(14):e00376-17. doi: 10.1128/AEM.00376-17. Print 2017 Jul 15. Appl Environ Microbiol. 2017. PMID: 28500039 Free PMC article.
References
-
- Alaedini A and Green PH (2005) Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med 142 289-298. - PubMed
-
- Arentz-Hansen H, Körner R, Molberg O, Quarsten H, Vader W, Kooy YM, Lundin KE, Koning F, Roepstorff P, Sollid LM, et al. (2000) The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J Exp Med 191 603-612. - PMC - PubMed
-
- Baert FJ, D'Haens GR, Peeters M, Hiele MI, Schaible TF, Shealy D, Geboes K, and Rutgeerts PJ (1999) Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology 116 22-28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

