A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging
- PMID: 19218555
- PMCID: PMC2681390
- DOI: 10.1194/jlr.M800555-JLR200
A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging
Abstract
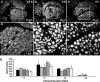
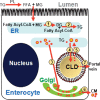
The absorptive cells of the small intestine, enterocytes, are not generally thought of as a cell type that stores triacylglycerols (TGs) in cytoplasmic lipid droplets (LDs). We revisit TG metabolism in enterocytes by ex vivo and in vivo coherent anti-Stokes Raman scattering (CARS) imaging of small intestine of mice during dietary fat absorption (DFA). We directly visualized the presence of LDs in enterocytes. We determined lipid amount and quantified LD number and size as a function of intestinal location and time post-lipid challenge via gavage feeding. The LDs were confirmed to be primarily TG by biochemical analysis. Combined CARS and fluorescence imaging indicated that the large LDs were located in the cytoplasm, associated with the tail-interacting protein of 47 kDa. Furthermore, in vivo CARS imaging showed real-time variation in the amount of TG stored in LDs through the process of DFA. Our results highlight a dynamic, cytoplasmic TG pool in enterocytes that may play previously unexpected roles in processes, such as regulating postprandial blood TG concentrations.
Figures




References
-
- Tso P., J. Balint, M. Bishop, and J. Rodgers. 1981. Acute inhibition of intestinal lipid transport by Pluronic L-81 in the rat. Am. J. Physiol. 241 G487–G497. - PubMed
-
- Phan C. T., and P. Tso. 2001. Intestinal lipid absorption and transport. Front. Biosci. 6 d299–d319. - PubMed
-
- Mansbach II C. M., and F. S. Gorelick. 2007. Development of physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomirons. Am. J. Physiol. Gastrointest. Liver Physiol. 293 G645–G650. - PubMed
-
- Christensen N. J., C. E. Rubin, M. C. Cheung, and J. J. Albers. 1983. Ultrastructural immunolocalization of apolipoprotein B within human jejunal absorptive cells. J. Lipid Res. 24 1229–1242. - PubMed
-
- Hamilton R. L., J. S. Wong, C. M. Cham, L. B. Nielsen, and S. G. Young. 1998. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J. Lipid Res. 39 1543–1557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

