Early synaptic defects in tulp1-/- mice
- PMID: 19218615
- PMCID: PMC3021947
- DOI: 10.1167/iovs.08-3190
Early synaptic defects in tulp1-/- mice
Abstract
Purpose: Mutations in the photoreceptor-specific tubby-like protein 1 (TULP1) underlie a form of autosomal recessive retinitis pigmentosa. To investigate the role of Tulp1 in the photoreceptor synapse, the authors examined the presynaptic and postsynaptic architecture and retinal function in tulp1(-/-) mice
Methods: The authors used immunohistochemistry to examine tulp1(-/-) mice before retinal degeneration and made comparisons with wild-type (wt) littermates and retinal degeneration 10 (rd10) mice, another model of photoreceptor degeneration that has a comparable rate of degeneration. Retinal function was characterized with the use of electroretinography.
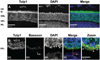
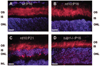
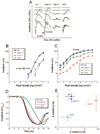
Results: In wt mice, Tulp1 is localized to the photoreceptor synapse. In the tulp1(-/-) synapse, the spatial relationship between the ribbon-associated proteins Bassoon and Piccolo are disrupted, and few intact ribbons are present. Furthermore, bipolar cell dendrites are stunted. Comparable abnormalities are not seen in rd10 mice. The leading edge of the a-wave had normal kinetics in tulp1(-/-) mice but reduced gain in rd10 mice. The b-wave intensity-response functions of tulp1(-/-) mice are shifted to higher intensities than in wt mice, but those of rd10 mice are not.
Conclusions: Photoreceptor synapses and bipolar cell dendrites in tulp1(-/-) mice display abnormal structure and function. A malformation of the photoreceptor synaptic ribbon is likely the cause of the dystrophy in bipolar cell dendrites. The association of early-onset, severe photoreceptor degeneration preceded by synaptic abnormalities appears to represent a phenotype not previously described. Not only is Tulp1 critical for photoreceptor function and survival, it is essential for the proper development of the photoreceptor synapse.
Figures





References
-
- tom Dieck S, Brandstätter JH. Ribbon synapses of the retina. Cell Tissue Res. 2006;326(2):339–346. - PubMed
-
- Schmitz F, Königstorfer A, Südhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28(3):857–872. - PubMed
-
- Morgans CW. Presynaptic proteins of ribbon synapses in the retina. Microsc Res Tech. 2000;50(2):141–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

